All published articles of this journal are available on ScienceDirect.
Eight Weeks of Interval Training Led to no Improvement in Cardiovascular Variables in the Elderly
Abstract
Background:
Interval training is a method with high acceptance in prescription to increase health and can be an essential intervener in improving cardiovascular function.
Objective:
This study aimed to verify the effects of eight weeks of interval training with different intensities on hemodynamic and autonomic function, which were assessed through resting heart rate, blood pressure, dual product, and heart rate variability.
Methods:
The sample consisted of 24 older men (age: 68.8 ± 6.8 years, body mass: 74.4 ± 18.1 kg, height: 1.70 ± 0.8 m; BMI: 25.1 ± 2, 2) who were physically active. Participants were randomized into 3 experimental groups: training group A (TGA, n = 8), training group B (TGB, n = 8) and control group (CG, n = 8). For trained groups, interventions were developed twice a week for eight weeks, with an interval of 48 hours between the sessions. The evaluations were carried out at the pre (baseline) and after the eighth week of intervention. The control group did not perform any intervention. The variables were analyzed for 10 minutes with subjects at rest in the sitting position before and after the intervention. Statistics with a significance level of p <0.05 were applied.
Results:
After the intervention, no statistically significant results were found in the variables assessed (p> 0.05).
Conclusion:
The intervention was not sufficient to promote statistical differences in hemodynamic and autonomic variables.
1. INTRODUCTION
Statistics show a significant increase in the life expectancy of the elderly, and the projection is that this population will continues to increase in the upcoming years [1, 2]. Studies related to disease prevention and health promotion make recommendations regarding the importance of physical exercise in the aging process [3]. This is because there is a concern regarding improving the health and quality of life of the world's elderly population [4, 5]. Physiologically, aging promotes inevitable changes throughout the body, which are considered mainly deleterious and influential in senescent health [6]. With the aging process, one of the most affected systems is the one that undergoes drastic changes such as the cardiovascular [7]. In this sense, to minimize and control these physiological losses, physical exercise is a primary way to promote healthier aging [8] and a better cardiovascular function [9].
Given the above, some aspects are indispensable, among which is the physical fitness index [10]. In the context of the cardiovascular system, the level of physical training defines the efficiency of this system [11, 12]. In the elderly, this whole process is significant for a better physiological reaction [13]. Thus, in aging, the practice of physical exercise has considerable importance for the promotion of better cardiovascular function, which is hemodynamic and also autonomic [14]. The hemodynamic and autonomic evaluation demonstrates the cardiovascular health of an individual, being decisive in the prevention of cardiovascular disorders [15, 16].
Physical exercise is essential to improve the cardiovascular markers of individuals, even in elderly [17]. Interval training (IT) is a positive way of improving hemodynamic efficiency [18] and autonomic modulation [19], thereby improving cardiovascular health parameters [20]. Studies applying IT considering the elderly, and intending to assess cardiovascular functions, are scarce, and therefore little is known about the dose-response in terms of intervention time, volume, and intensity.
This training method can be proven effective for this population, since it allows for shorter execution times, having higher impact, which can be better when compared to other methods because it offers less risk of overload and more comfortable practice for individuals with motor conditions [21, 22]. Therefore, this study aimed to verify the effects of eight weeks of interval training. Different intensities on hemodynamic and autonomic function were assessed through evaluating resting heart rate (HRR), Systolic Blood Pressure (SBP) and Diastolic Blood Pressure (BPD), Mean Arterial Pressure (MAP), Double Product (DP), and Heart Rate Variability (HRV). The hypothesis was that interventions using protocols with different intensities would be able to promote significant improvements in these variables when compared to the control group.
2. METHODS
2.1. Participants
The sample consisted of 24 older men (age: 68.8 ± 6.8 years, body mass: 74.4 ± 18.1 kg, height: 1.70 ± 0.8 m; BMI: 25.1 ± 2.2) being physically active (Table 1). Inclusion criteria were older men, who were regular practitioners of physical activity, except for aerobic exercise, and having minimal physical conditions to perform the proposed intervention by the study. The exclusion criteria were the use of drugs or ergogenic resources that could influence the expected results in some way, and presenting muscle disorders that can compromise the training. After explaining the risks and benefits of the research, the subjects filled out the Physical Activity Readiness Questionnaire (PAR-Q). They signed a free and informed consent form following Resolution 466 of 2012, of the National Health Council (BRAZIL, 2016), and the Helsinki Resolution.
2.2. Experimental Design
Participants were randomized into 3 experimental groups: training group A (TGA, n = 8), training group B (TGB, n = 8) and control group (CG, n = 8). For the trained groups, the interventions were developed for 16 sessions, with 2 to 3 sessions for 48 hours between one and the other session. The evaluations were performed at the pre (baseline) and after the eighth week of intervention. The control group did not perform any intervention and continued with their daily household activities, but without performing activities such as walking, cycling, or other activities that could interfere with the already existing fitness index in these individuals. However, they performed the assessments in the same period as the training group.
2.3. Analysis of Cardiovascular Variables
For the hemodynamic variable, HRR, SBP, and DBP values were analyzed. Blood Pressure (BP) values were collected from the left arm [23, 24]. The variables were analyzed in 10 minutes with the subjects at rest and in a sitting position. For HRR, SBP, and DBP, the average of the 8th and 9,th minute of measurement was used. After determining BP data, MAP was calculated using the formula MAP = SBP + (DBP X 2) / 3. Then, using HRR and SBP, the double product (DP) was calculated using the equation: [HR (bpm) X SBP (mmHg)]. The DP represents the heart's workload or oxygen demand and is considered a non-invasive reference for cardiac overload [25].
For autonomic analysis, the behavior of HRV was used, measured in a window of 5 minutes to 10 minutes with the individual, also, in a sitting position. The mean and time-domain indices (RR, RMSSD, and SDNN) and frequency (LF, HF, and LF/HF) were considered. The temporal domain was standard RR (time between heartbeats and others). Statistical or geometric methods were employed (mean, standard deviation, and indexes derived from the histogram or the cartesian coordinate map of RR intervals). The indexes translators of fluctuations in the duration of the cardiac cycles were calculated, being RMSSD (square root of the square mean of the differences between the adjacent standard RR intervals, in a time interval, expressed in ms) and the SDNN (standard deviation of all RR normal) intervals recorded in a time interval, shown in ms.
The RMSSD represents parasympathetic activity, and the SDNN represents sympathetic and parasympathetic activity. Still, it does not allow us to distinguish when changes in HRV are due to the increase in sympathetic tone or the removal of vagal tone.
For the analysis of HRV in the frequency domain, low-frequency components (Low Frequency - LF) were used, which correspond to the joint action of the parasympathetic and sympathetic in the heart with a predominance in the sympathetic and high-frequency component (High Frequency - HF), corresponding to a respiratory modulation and representing the activation of the vagus nerve. Finally, we used the LF / HF ratio that represents sympathetic-vagal balance [26, 27].
A POLAR RS800CX® watch (Multisport, Kempele, Finland) ™ [28] was used to collect HR and HRV. For the analysis of BP, a digital oscillometric device from the brand OMRON M6® (HEM-7001- E) ™ [29] was used. For the treatment of HRV, the data were transferred to the computer and attached to Polar Trainer 5 Software®. Correction procedures for all data were performed on this platform and subsequently filed in TXT format for the start of treatment in Kubios HRV Standart Software®, version 3.3.1. In this sense, all the data collected were calculated and presented in different standards so that there are broad interpretations concerning HRV.
2.4. Training Protocols
The interval training protocol was performed twice a week, for eight weeks, for both experimental groups, but with different intensities. The intensities were controlled through calculations based on the maximum heart rate (HRmax), adjusted with the reserve heart rate [30]. The TGA was performed for 4 minutes with intensity relative to 55 to 60% of HRmax and 1 minute to 70 to 75% of HRmax. The TGB training group, in turn, performed the same protocol but for 4 minutes at 45 to 50% of HRmax and 1 minute at 60 to 65% of HRmax. In the two training groups (A and B), each four by one sequence was considered a block, representing a total of 6 blocks. The duration was equivalent to thirty minutes. At the end of each block, the PSE was measured [31] to assist in the proposed intensity control [32]. All training sessions were carried out on a treadmill under the MOVEMENT® (RT 250) ™ brand.
2.5. Statistical Analyses
In the descriptive analysis, the means and standard deviation of the autonomic and cardiovascular variables were calculated. Normality was not rejected by the Shapiro-Wilk test, as well as by the analysis of the histogram and Q-Q Plot and homoscedasticity was confirmed by the Mauchly test'. This suggests a normal distribution of the collected data implying the possibility of parametric inferential treatment. The analysis of variance (ANOVA) with repeated measures was applied to test the main and interaction effects. Tukey's test for multiple comparisons was applied to observe inter and intragroup comparisons. All statistical analyses were performed using SPSS software version 21 (SPSS Inc., Chicago, IL, USA), with a significance level of 5% (p < 0.05). To calculate the sample size, we used the GPower 3.1 software.
3. RESULTS
Twenty-four old-age people were selected (Table 1), and were randomly assigned to TGA, TGB, and CG. Each group comprised of 8 participants; all the selected participants completed the interventions or evaluations determined or both.
Table 1. Anthropometric characteristics and baseline variables of the participants.
There was no interaction between groups for any of the variables evaluated (p> 0.05). For hemodynamic analysis, HRR, SBP, DBP, MAP, and DP were used. For HRR (Fig. 1), there was no interaction between groups (p = 0.9127). Likewise, there was no significant difference in the comparison before and after intervention for TGA (p > 0.9999), TGB (p = 0.9991) and CG (p > 0.9999).
Regarding SBP (Fig. 2A) and DBP (Fig. 2B), there were also no interactions between groups (p = 0.0775 and p = 0.979, respectively). With regard to pre- and post-intervention comparisons, no significant difference was observed for any group. The SBP and DBP vslues for the groups were as follows; TGA (p = 0.7686 and p > 0.9999, respectively), TGB (p = 0.4416 and p > 0.9999, respectively) and CG (p = 0.8210 and p = 0.9970, respectively). Additionally, MAP (MAP = SBP + (DBP X 2) / 3) and DP (HR X SBP) were calculated. Regarding MAP (Fig. 2C), there was no interaction between groups (p = 0.954), as well as no significant difference was observed before and after intervention for TGA (p = 0.8646), TGB (p = 0.5330) and CG (p = 0.8030).
Regarding the DP (Fig. 3), the results followed the same direction. There was no interaction between groups (p = 0.8135) and there was no significant difference before and after intervention for any of the groups (TGA: p = 0.9873, TGB: p = 0.9988 and CG: p = 0.9999).
| Variables | TGA | TGB | CG | |
|---|---|---|---|---|
| - | M±DP | M±DP | M±DP | |
| Age (years) | 65.1 ± 4.3 | 73.1 ± 7.2 | 68.2 ± 6.6 | |
| Weight (kg) | 81.9 ± 13.1 | 74.2 ± 7.3 | 75.8 ± 5.2 | |
| Height (m) | 1.71 ± 0.06 | 1.69 ± 0.06 | 1.71 ± 0.04 | |
| BMI (kg/m2) | 27.8 ± 1.4 | 25.8 ± 1.2 | 26.8 ± 1.4 | |
| HRR (BPM) | 73 ± 11 | 76 ± 14 | 78 ± 7 | |
| SBP (mm/Hg) | 129 ± 5 | 128 ± 10 | 126 ± 9 | |
| DBP (mm/Hg) | 80 ± 3 | 77 ± 6 | 80 ± 4 | |
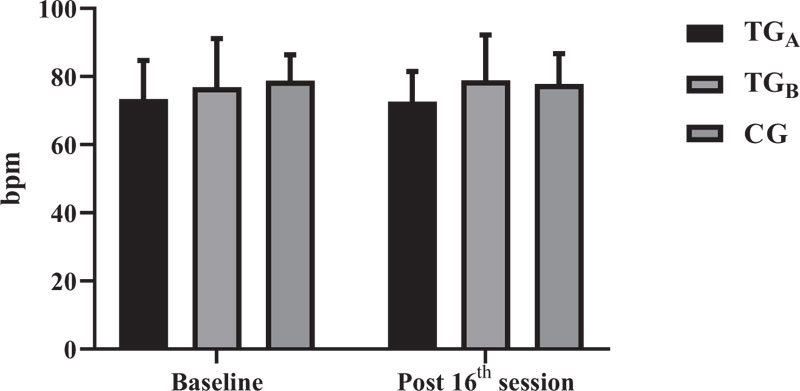
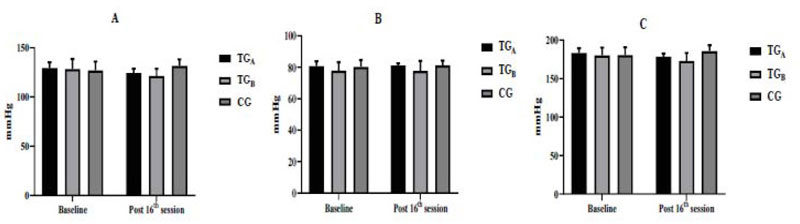
Regarding the autonomic evaluation, HRV was used with an analysis of the indexes in the time domain (RR, RMSSD, and SDNN) and in the frequency domain (LF, HF, and LF / HF). There were no interactions between groups for any of the indexes evaluated, being RR (p = 0.9754), RMSSD (p = 0.9376), SDNN (p = 0.8054), LF (p = 0.4185), HF (p = 0.6076) and LF / HF (p = 0.1876). Regarding the pre and post-intervention analyses, there was also no significant difference in the indices for TGA, TGB, and CG (p > 0.05). The values of the HRV indices before and after intervention are shown in Table 2.
Table 2. Heart rate variability in the time and frequency domain, pre and post-intervention for TGA, TGB, and CG in mean and standard deviation (m / Sd).
| Index | TGA | TGB | CG | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | p | Pre | Post | p | Pre | Post | p | |
| RR (ms) | 832.11±127.02 | 850.75±90.05 | 0.9992 | 825.82±143.43 | 848.50±114.83 | 0.9978 | 768.45±50.37 | 775.33±64.16 | >0.9999 |
| RMSSD (ms) | 16.82±10.39 | 14.65±5.06 | 0.9999 | 24.33±26.54 | 23.82±27.69 | >0.9999 | 22.75±12.94 | 25.06±11.85 | 0.9998 |
| SDNN (ms) | 40.72±13.59 | 39.85±15.61 | >0.9999 | 42.15±30.58 | 52.82±35.58 | 0.9552 | 49.18±19.96 | 55.83±27.64 | 0.9947 |
| LF (n.u) | 73.08±28.08 | 69.28±28.11 | 0.9990 | 83.60±6.67 | 75.82±12.47 | 0.9737 | 74.88±19.58 | 85.60±19.81 | 0.9022 |
| HF (n.u) | 26.97±28.04 | 30.68±28.12 | 0.9990 | 16.40±6.67 | 22.96±10.06 | 0.9850 | 25.11±19.58 | 18.32±15.67 | 0.9826 |
| LF/HF (ratio) | 6.22±4.72 | 4.69±4.01 | 0.9631 | 5.79±2.02 | 4.61±3.80 | 0.9883 | 5.45±4.31 | 5.46±3.02 | >0.9999 |
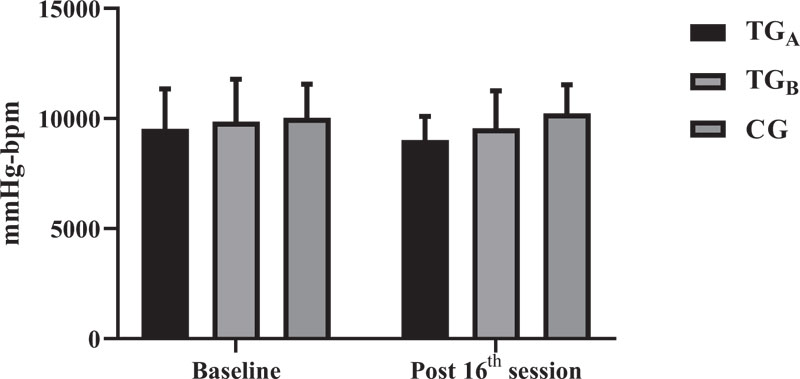
4. DISCUSSION
According to global perception [33], functional abnormalities in cardiovascular variables are the leading causes of illness and death in the world, especially in the elderly [34]. This study aimed to verify the effects of eight weeks of interval training with different intensities on hemodynamic and autonomic function. Hemodynamic variables (HRR, SBP, DBP, MAP, and DP) and autonomic variables (HRV) were assessed. The intervention was performed, comprising eight weeks of interval training for two training groups (TGA and TGB). The CG did not carry out the practice, but the evaluations of the group were carried out in the same period as the experimental groups.
The TGA performed the IT protocol which comprised of six series of 4 minutes at 55-60% of HRmax and 1 minute at 70-75% of HRmax. The TGB performed the same number of series, but 4 minutes at 45-50% of HRmax with 1 minute at 60-65% of HRmax; therefore, the TGB performed interventions with lower intensities. Upon completing the 16 sessions proposed by the study, the results demonstrated that both training groups achieved similar results, with no significant differences between the groups. (p > 0.05). In the same sense, the intragroup results also did not show significant differences (p > 0.05) for all variables evaluated. The organization of analyses and interventions in the present study was similar to that of Pichot et al. [17]. However, in that research, the authors performed the intervention with only one group, for 14 weeks, having four sessions per week and the protocol was performed on a cycle ergometer containing nine series of 4 minutes at 65% of HRmax and 1 minute at 85% of HRmax, totaling a volume of 45 minutes. In the present study, the interventions were carried out on a treadmill, corresponding to 32 sessions distributed in 3 weekly sessions. The recovery time (4 minutes) and stimulation time (1 minute) were the same as Pichot et al. [17]; however, the intervention was carried out in 6 series, totaling 30 minutes of training, and in 3 different groups, two training (TGA and TGB) and one control (GC).
In the hemodynamic analysis, studies intervened with IT observed positive results. Pichot et al. [17] demonstrated improvements in HRR, SBP, DBP, and MAP (p < 0.05). Molmen et al. [22] applied IT to active and sedentary older people and observed improvements in HRR, SBP, and BPD (p < 0.05) after 12 weeks of intervention with three weekly sessions. Nemoto et al. [21] managed to improve the HRR, SBP, and DBP of women after five months of intervention with IT using walking as an activity, 2 to 4 times a week. Regarding autonomic analysis, it seems that IT is an efficient method to promote improvements. In addition to the study by Pichot et al. [17], other experiments also obtained positive responses in HRV (p < 0.05) after the intervention with IT [19, 20]. In the present study, we chose to control session time through the number of sessions performed, but not less than two and more than three sessions per week. According to the studies mentioned above, it seems that a longer total intervention time is an indispensable factor for improving cardiovascular functions. Regarding the number of sessions per week, the study conducted by Leprete et al. [35] like the present study, did not achieve hemodynamic improvements in a period of two times a week, even applying nine weeks of intervention and demonstrating that the number of weekly sessions can also directly interfere with cardiovascular responses. The present study evaluated DP and MAP, and it appears that these variables are dependent on improvements in HRR and BP [17]; however, our findings followed the behavioral logic of these variables.
Regarding intensity, it seems that high stimulus intensities can improve hemodynamic functions in men and women with two sessions per week [6]. However, the results regarding the stimulus intensity in IT intervention for the elderly are still inconclusive. Studies show positive responses with work intensities below the threshold limits [20]. Also, high-intensity stimuli show levels above the metabolic or ventilatory limits, or both [36]. In this study, the IT intervention was performed in two groups (TGA and TGB), with different intensities, 70-75% of HRmax (TGA), and 60-65% of HRmax (TGB). The aim was to investigate whether a short time and mild to moderate to strong intensities, could have positive results in hemodynamic and autonomic responses. In this way, IT could be effectively used as a method for improving the health of the elderly without posing any risks or health hazards as many older people are restricted to withstand high intensities for a given stimulus time. Our objective was to find out if the 8-week intervention would be able to cause positive changes in the hemodynamic and autonomic behavior of active older people, using interval training at different stimulus intensities (70-75% of HRmax and 50-65% of HRmax, TGA and TGB, respectively). Thus, we could suggest that, for this population, lower intensities could also generate positive responses, especially in HR, HRV, and BP. However, for these intensities, perhaps a longer intervention time is adequate for the appearance of significant improvements in these variables. Hence, we did not obtain statistically significant results.
The possible mechanisms for responses obtained in the elderly are still uncertain, but plausible. Some cardiovascular adaptations can be affected in the elderly, such as central and peripheral functions. The aerobic stimulus can reduce the plasma level of renin, reflecting the decrease of the renin-angiotensin system, improving the baroreflex activity, and consequently, the behavior of BP, HRR, and HRV [17-19]. The improvements in capillary density, endothelial function, and oxygen delivery to tissues are factors that can influence hemodynamic and autonomic development [6-21-37]. Also, it may help to improve plasma hemoglobin and myoglobin volumes in muscles and increase muscle capacity, positively impacting the entire cardiovascular system [35]. All these physiological functions are more difficult in the elderly due to the aging process, being directly dependent on the level of physical conditioning; therefore, it can be concluded that regular physical exercise is essential [9-11].
The present study has some limitations, which may have influenced the results obtained. The number of participants could have been higher, but, due to the difficulty of finding healthy older people available for research, this factor hindered the objective. The sample size calculation determined 12 participants for each group, but due to the difficulty of finding healthy older people available for research, 8 participants were selected for each group. We could have performed the intervention on two groups (1 training group and one control group); in this way, appropriate sample according to the calculation could have been obtained. But our objective was to compare different exercise intensities, between different training groups, in addition to the control group, to demonstrate the potential of each level of intensity and, thus, to provide possible information for prescriptions in this population.
Another possible limitation is the stimulus time of 1 minute for the intensities used in the training group, which may not have been sufficient to generate physiological adaptations in the cardiovascular system. The purpose of the study was to make all older people perform three sessions per week, but taking into account the participants' requirements, this objective was difficult to achieve. It was determined then that it could not be less than two times a week, but we recognize that this is a limiting factor of the study.
CONCLUSION
The proposed intervention was not sufficient to promote statistical differences in hemodynamic and autonomic variables. As the subjects were from the elderly population, it seems that this population needs an intervention with higher frequency per week, longer intervention time, and perhaps higher intensities for the observation of more relevant results in terms of statistical comparison. Therefore, it is essential to emphasize that for performing a training intervention with the elderly, it is necessary to evaluate each individual regarding training needs taking into account intensities, volumes, and even longer intervention times.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study complied with Resolution 466/12 of the National Health Council (CNS). The study was approved by the local Research and Ethics Committee of the Salgado de Oliveira University, Rio de Janeiro, Brazil, registered with the protocol: CAAE: 48827415.8.0000.5289.
HUMAN AND ANIMAL RIGHTS
No animals were used in this study. All human research procedures performed in the current study followed the ethical standards of the institutional and national research committee and the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
CONSENT FOR PUBLICATION
All participants were informed about the protocol and their written informed consents were obtained before participating in the study.
AVAILABILITY OF DATA AND MATERIALS
The datasets analyzed during the current study are available from the corresponding author [Sant'Ana LO] on immediate request.
FUNDING
Sergio Machado was supported by a grant from Carlos Chagas Foundation for the Research Support in the State of Rio de Janeiro (FAPERJ), Young Scientists from the State of Rio de Janeiro, E -26/203.295/2017.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
The Author acknowledges the Federal University of Juiz de Fora, Minas Gerais, Brazil for providing scholarship to the researcher and Ph.D. student in Physical Education Leandro de Oliveira Sant'Ana.


