All published articles of this journal are available on ScienceDirect.
Application of Theory in Chronic Pain Rehabilitation Research and Clinical Practice
Abstract
Introduction:
Chronic pain has multiple aetiological factors and complexity. Pain theory helps us to guide and organize our thinking to deal with this complexity. The objective of this paper is to critically review the most influential theory in pain science history (the gate control theory of pain) and focus on its implications in chronic pain rehabilitation to minimize disability.
Methods:
In this narrative review, all the published studies that focused upon pain theory were retrieved from Ovoid Medline (from 1946 till present), EMBAS, AMED and PsycINFO data bases.
Results:
Chronic pain is considered a disease or dysfunction of the nervous system. In chronic pain conditions, hypersensitivity is thought to develop from changes to the physiological top-down control (inhibitory) mechanism of pain modulation according to the pain theory. Pain hypersensitivity manifestation is considered as abnormal central inhibitory control at the gate controlling mechanism. On the other hand, pain hypersensitivity is a prognostic factor in pain rehabilitation. It is clinically important to detect and manage hypersensitivity responses and their mechanisms.
Conclusion:
Since somatosensory perception and integration are recognized as a contributor to the pain perception under the theory, then we can use the model to direct interventions aimed at pain relief. The pain theory should be leveraged to develop and refine measurement tools with clinical utility for detecting and monitoring hypersensitivity linked to chronic pain mechanisms.
1. INTRODUCTION
“From the brain alone arise our pleasures, laughter, and jests, as well as our sorrows, pain, and griefs.” Hippocrates. Pain is an ancient topic and our thinking on the nature of pain has been shifted over the centuries from the Cartesian dualistic concept to the Gate Control Theory (GCT) of Pain, a better global model of pain. It is difficult to define chronic pain due to its complex nature. The need for a better definition was emphasized by John Bonica [1], who referred to the diverse taxonomies in use as “the tower of Babel”. The International Association for the Study of Pain (IASP) has led efforts to establish a common taxonomy: it defines chronic pain as a pain syndrome lasting for more than 3 months [2]. Chronic pain has multiple aetiologies, including chronic diseases like arthritis; acute injuries with lingering symptoms after fracture [3]; or can persist following major surgery. The incidence of chronic pain in the general population is estimated at 20% to 50% [4]. According to the National Institute of Health, pain affects more people than diabetes, heart disease, and cancer combined [5, 6]. The economic costs of pain have been estimated as being more than $100 billion yearly in United States [5, 6]. In 2010, the IASP recognized chronic pain as a serious global chronic health problem with a huge economic impact [7]. The IASP identified chronic pain as a highly stigmatized condition regardless of associated diagnoses, and highlighted the need for appropriate assessment to reduce burden [7]. As a signal of its importance, pain is now considered as the 5th vital sign [7, 8], despite complexity in the nature of pain.
Pain is a protective mechanism that ideally warns of imminent tissue damage. However, in some cases, the organism can be too sensitive (hypersensitive) and perceive pain where no tissue damage is imminent, or where the stimulus intensity is below the normal threshold necessary to elicit a pain response [9]. Pain hypersensitivity, used here as an umbrella term incorporating both allodynia and hyperalgesia (Table 1), manifests as a spread of increased somatosensory responsiveness into adjacent normal tissues, even after the end of a noxious (painful) stimulus (e.g. in nociplastic pain) [10]. Evidence synthesis indicates strong evidence of hypersensitivity (abnormal pain response) as a prognostic factor for poor outcomes in chronic musculoskeletal pain [11]. There is increasing awareness that chronic pain is, at least in part, a disease of the nervous system [9, 12]. Thus, it is important to have a conceptual framework to interrogate and interpret how the nervous system contributes to pain.
| Topic | Allodynia | Hyperalgesia |
|---|---|---|
| IASP Definition | Pain due to a stimulus that does not normally provoke pain. | Increased pain from a stimulus that normally provokes pain. |
| Pain mechanism | Lowered threshold | Increased response |
| Stimulus and response mode | Differ | same |
Note: The table is reused with permission from Uddin Z and MacDermid JC. Pain Studies and Treatment. 2014;2(2):31-35.
Theory helps us to guide and organize our thinking to deal with the complexity of pain by explicating our assumptions and systematically testing relationships. Existing pain theories are capable of explaining some aspects of pain, but there is no comprehensive theory or model that we can use to interpret or explain all aspects of pain [13, 14]. Theory can help us frame our understanding of complex phenomena like pain, and to interpret what we observe in clinical practice [15]. In pain science, theories help determine how we study neurophysiology, how we interpret the observations from pain bench or lab studies; and how we treat patients with pain [16, 17]. Pain mechanisms are not yet completely understood. Nonetheless, the widely accepted GCT holds some testable propositions to explain the pain. The purpose of this paper is to highlight the importance of theory (as an example of the most influential GCT in the pain science history) and explain its implications in chronic pain rehabilitation, focusing on both research and clinical practice.
2. ROLE OF THE GCT
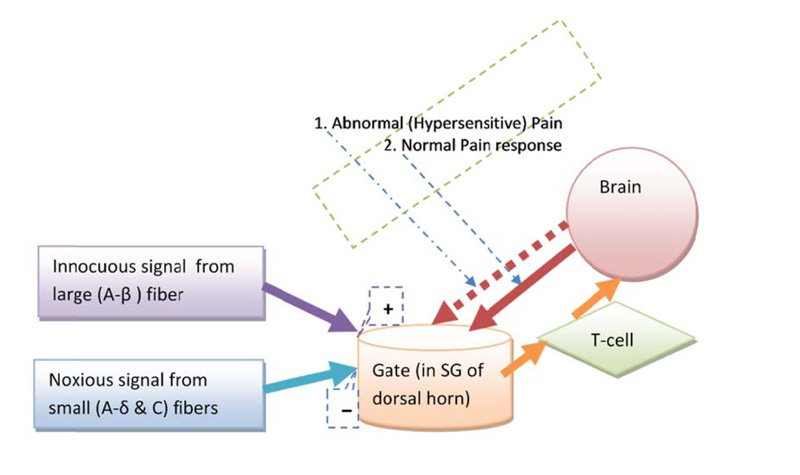
Melzack and Wall (in 1965), proposed a “gate” system within the dorsal horn of the human spinal cord, which regulates pain perception by a dynamic inhibitory-facilitatory mechanism [18]. GCT consists of four basic structural components (Fig. 1): small fibers (A-delta and C fiber), large fiber (A-beta nerve fiber), substantia gelatinosa (SG, lamina-II of dorsal horn), and the hypothetical first central transmission cell. The theory explains the relationship between these four components. The small and large nerve fibers project toward the SG of lamina-II and first central transmission cell. The modulatory (inhibitory) effect on pain processing is increased in SG by activation of large fibers and decreased by small fibers activity. A diagrammatic representation of the GCT (Fig. 1) shows the gate control system in the dorsal horn receiving feedback from the central control, and reflecting the central inhibitory influence of pain [18]. The central inhibitory influence plays a major role in both hypersensitivity and chronic pain mechanisms [19-23].
GCT explains the role of the central nervous system (both spinal cord and brain) in the perception of acute and chronic pain conditions [24]. The gate models how a peripheral stimuli interact to modulate pain sensation and perception. The theory explains the role of A-beta fibers, pain fibers (i.e. A-delta and C fiber), and the dorsal horn of the spinal cord for central transmission of pain perception [25]. It reflects physiologic mechanisms (e.g. central summation of pain stimulus, and other somatosensory inputs) into the control of pain. The theory helped us thinking about how pain generates away from peripheral nervous system into the central nervous system with central summation and somatosensory input and contributions to our pain perception. Fundamentally, two opposing gate controlling mechanisms (inhibition and facilitation) in GCT are key factors of pain modulation. In chronic pain scenario, hypersensitivity phenomena is thought to develop from physiological changes to the inhibitory component in the GCT [19-23]. From a GCT perspective, the clinical manifestation of hypersensitivity is considered to represent central abnormal inhibitory control within the gate control mechanism (Fig. 1).
The theory explains pain regulation in a way that can account for both abnormal (hypersensitive or hyperpathic) and normal (physiological) pain responses. The theory hypothesized pain sensation (nociception) is regulated dynamically in the dorsal horn, which can lead to a decrease or increase pain sensitivity [11]. A noise in the inhibitory controlling mechanism is thought of as one of the key causes of developing hypersensitivity [19-23]. Hypersensitivity is a reflection of imbalance within the two opposing neurophysiological pain modulatory activities, such as, gate control or top-down control (Fig. 2).
3. RESEARCH FINDINGS SUPPORTING THE GCT
In 1965, the original paper proposed the theory in light of spinal neurophysiologic observations. Subsequently, the authors extended the scope of the GCT views to include other components in the central nervous system [24, 26]. Even the revised format retains simplicity in how it portrays the neurophysiologic basis of pain [26]. The characteristics of a good theory (e.g. accuracy, consistency, scope, simplicity, and fruitfulness) are reflected in studies that support the GCT [26]; and how this theory helps to address problems [18, 26, 27].

Since the inhibitory pathway play an important role in pain hypersensitivity, research support for that aspect of the model is focused. The GCT suggested a role of inhibition by endogenous mechanisms of pain control [28]. An experimental study showed that repeated electrical stimulation in peripheral nerve fibers can produce electro-analgesia [29], which was consistent with expectations based on the GCT. Further evidence of the accuracy of the model came from the discovery of endogenous inhibitory circuits [30]. These findings were consistent as they were replicated by several independent studies showing that pain hypersensitivities are produced by interrupted pain inhibitory mechanisms in the spinal cord; [31-38] also studies showing that improving inhibition can reduce hypersensitivity [39-42].
Britton and colleagues [43] took a unique approach to evaluate the accuracy of the GCT by creating a mathematical model from the concept of the theory and then testing whether the resultant mathematical computations fit the observations in existing research. The mathematical model of GCT [43] supported that the large fibers connected to the higher brain (cognitive control mechanism) via dorsal column can modulate pain by inhibition or facilitation. The mathematical model also supported the theory in that it was a robust model that explained a number of different pain phenomena. However, this modelling also demonstrated short falls where it did not adequately explain all observations (i.e. stimulation of large fiber doesn’t reduce pain always).
Patrick Wall [26] noted that although the mechanism by which the gate control achieved is not fully understood, the model does support continued investigation of the functional role and mechanisms, demonstrating that it is considered “fruitful” in supporting pain research. Finally, support for the GTC is demonstrated by its longevity [17]. Since proposed in 1965, there have been minor changes; but basically, no major opposition has been raised by a competing theory.
4. CONTROVERSY AND GAPS IN GCT
There are some limitations anomalies in the GCT. Some studies with humans did not identify evidence of the gating mechanism in the spinal cord by measuring cutaneous sensory stimulations and its impact on electrical recordings from non-myelinated fibers [44-49]. According to the GCT of pain, the large fiber stimulation should inhibit pain. However, Nathan and Rudge [50] stimulated large fiber in humans; and did not find reductions in pain. They suggested that some essential parts of GCT might be wrong. The critical review of GCT by Nathan [51] described the limitations of the antagonist relationship between the large and small fibers; and how the theory would collapse if the antagonist relationship fails.
There are gaps where the GTC is not able to explain phenomena [50]. One such identified gap is the GCT does not address stimulus specificity (e.g. thermal, mechanical, electrical and chemical) in the peripheral nervous system. Another gap is the theory is overly simplistic and does not clarify whether the facilitation and inhibition are pre or post-synaptic, or both [51]. Pre-synaptic facilitation and inhibition in GCT remain a debated issue, although it has remained difficult to isolate the role of pre-synaptic and postsynaptic fibers.
The GCT does not explain the T-cell (first central transmission cell) as a specific central connecting neuron or tract, but assumes the presence of a wide dynamic range neuron that connects to multiple areas within the brain. However, functional and anatomical findings suggested the existence of a specific labeled line of central pain pathways [52]. The GCT does not specify the location of T-cell, but it assumes as the high-frequency signal toward the midbrain [43]. The theory cannot explain all types of pain (e.g. phantom limb pain, central post-stroke pain), but as our understanding of different pain phenomena continues to evolve [53], our understanding of the explanatory power of gate control will also evolve. The pain processing mechanism and response within the brain are not explained in detail by the GCT. Moreover, motor adaptation (sensory-motor control) and pain are not explained in theory.
After acknowledging these limitations, it is perhaps necessary to briefly look at the scope of the pain theory literature to situate GCT. Other pain theorists have focused on pain processing in the brain, postulating a ‘pain matrix’ to describe the interrelationships of neural centres activated by nocioceptive inputs from the periphery, which mark the emergence of the pain entity [54]. However, there is an acknowledgement that other brain centres not included in the ‘matrix’ ultimately modulate those signals, and influence the efferent responses. Hodges’ theory of motor adaptation in pain [55] further posits both biological and behavioural responses in the central nervous system and periphery as inherent to the pain experience. Considered in isolation, each of these theories fails to explain the entire spectrum of the pain phenomenon, but considered together, they expand our ability to understand the complexity and inform individualized treatment planning. Our intent herein was not to ignore the breadth of these contrasting and complimentary perspectives, but to explore the singular depth of GCT.
5. GCT USED IN PAIN RESEARCH
The GCT played a major role in the pain research direction and many influential scientific findings. It has been reported in a meta-trend analysis, which accounted pain research from 1975 to 2007 [56], and demonstrated the original GCT article [18] as the top most cited paper. The study was based on 4525 research papers published in the official journal of IASP (Pain) and illustrated the predominance of GCT in the field of pain research. The meta-trend analysis also identified chronic pain as the most common clinical condition, which should be studied keeping in view the hypersensitivity phenomena.
Within clinical pain interventional type of research, rehabilitation interventions have interpreted electrotherapies using the GCT lens (e.g. transcutaneous electrical stimulation). A recent bibliometric analysis from research studies [57], on rehabilitation interventions highlights the importance of this evidence. The analysis has demonstrated physical modalities as the most investigated topic [57]. It was based on an analysis of 2519 treatment-focused publications between 1980 -2009 in the “Physical Therapy” journal. This piece of evidence reflects the predominance GCT conceptual premise intervention (i.e. gate closure via A-beta fiber and pain inhibition) type of research in physical rehabilitation.
Over the last four decades, most of the novel discoveries in pain areas relied on the concept of GCT [58] and it remains a framework for promising new research areas. Current research trend areas where GCT is fundamental to the line of investigation include: diffuse noxious inhibitory control of pain (i.e. an endogenous pain modulatory pathway), hypersensitivity, and endogenous opioid receptors. Hypersensitivity is a specific area of the recent focus in rehabilitation research, in that pain typology is now being used to define treatment needs and to predict future outcomes. For example, there is a substantial body of work now showing whiplash patients who demonstrate early abnormalities in cold sensitivity have a poor prognosis and require different rehabilitation approaches [59]. Mechanical allodynia has been related to lowered adherence and poor prognosis in complex regional pain syndrome [60, 61], and emerging work is focusing on developing evidence for accurate assessment and treatment strategies [62-64].
6. GCT USED IN REHABILITATION PRACTICE
The tenets of GCT have also informed and shaped pain rehabilitation practice over four decades. A proposition of GCT explains neurophysiological consequences of large fiber stimulation and its output in pain modulation [18]. Rehabilitation therapy uses that principle to gain pain control by manipulating the fast conducting large fibers [18]. Physical agents/modalities, such as whirlpool, fluidotherapy, and massage among many other therapies are applied, which are based on principles of gaining pain relief by stimulating large fibers. For example, large fibers are stimulated by touch or gentle rubbing on skin [65, 66] and can be used strategically to reduce painful sensitivity [62, 67]. Further, in our understanding, the theory has an influential role on therapeutics electro-analgesic mechanism. Since the ancient time of Aristotle, electro-analgesic has been used to treat pain [68] and it was thought as a quackery practice. The GCT is a scientific foundation of electro stimulation based therapies and it was accepted by the medical community for pain relief soon after the publication of Wall and Sweet [27]. Later, endogenous opioids supported the chemical basis of using electrical stimulations and the explanatory model derived from GCT [69].
The theory has coincided with the recognition of therapy professions and their modalities. GCT has been integrated in pain science curriculum for therapists, which is helpful for better understanding rehabilitation interventions in pain. Many pain related clinical conditions mechanisms are poorly understood, but the therapist can use a theoretical rationale to explain pain from an unknown mechanism (i.e. phenomena like pain hypersensitivity, referred pain, etc.). It now underpins much of pain neuroscience education, which in itself has demonstrated the effect for pain management as part of a comprehensive approach to rehabilitation [70, 71].
Since the theory suggests that pain response and perception are triggered by sensory feedback and central integration via the dorsal horn, this can give us leverage to alter the pain perception by sensory input intervention. For example, desensitization uses graded exposure based therapy to different sensory inputs for modifying pain input [72], which would lead to reducing pain sensitivity via inhibitory mechanism. Interventions used in physical rehabilitation, including hands-on therapy, graded exercise result in sensory inputs via the “gate” in a way, which facilitates better pain control [54]. The theory can help rehabilitation professionals for extending their interventions into an innovative way to alter pain perception.
7. FUTURE DIRECTIONS
The GCT can explain pain hypersensitivity, which assist us in understanding some of the mechanisms at play in chronic musculoskeletal pain. Both researcher and clinician might be beneficial from theory-informed research and practice. Pain hypersensitivity responses after tissue injury are linked with the somatosensory signalling within the central nervous system [73]. Clinically, hypersensitivity manifests as two common but distinct abnormal pain responses (Table 1) either by lowering the pain threshold (allodynia) or increasing the pain response (hyperalgesia). These pain hypersensitivity phenomena have been found in different musculoskeletal pain conditions [9, 59, 74-83]. There is emerging evidence that early hypersensitivity is predictive of outcomes after injury, e.g. whiplash [59], and in chronically painful musculoskeletal conditions [11, 83]. However, we do not know if this extends to a number of upper extremity disorders, such as distal radius fracture, tenosynovitis, epicondylitis, and persistent post-operative pain after carpal tunnel release. Furthermore, much of this research has been conducted in controlled laboratory conditions and with devices not used in clinical practice making it difficult to move the findings into clinical practice. This underscores the need to continue to support the implementation of those devices and techniques validated with clinical populations and accessible for clinical use [84-98]. The GCT is a framework for understanding hypersensitivity in chronic pain. One of the critical next steps needed is reliable and valid psychophysical techniques for measuring hypersensitivity (pain sensitivity) and sensitivity to physical activity that can be operationalized in clinical settings using readily available testing equipment [99, 100].
CONCLUSION
Pain in humans is a multi-dimensional, complex sensation-perception that ultimately generates a huge burden to the society. It is affected by multidimensional factors (e.g. cognitive, emotional, and social). Many theories of pain have been proposed by scientists over the centuries, however, very few were accepted. The GCT has become the predominant theory with a resultant far-reaching impact on the understanding of pain mechanisms, providing a useful way for us to deal with the complexity of pain. The GCT stimulated an intense research interest and discovery in all branches of pain science for the last 50 years, due to the physiology-based, testable propositions of the theory.
Since somatosensory perception and integration are recognized as a contributor to the pain perception under GCT, then we can use the model to direct interventions aimed at pain relief. The theoretical concepts of pain from the gate control theory underpinning neurophysiology-based models of central integration continues to inform research and mechanism-specific management. Moving forward, the pain theory should be leveraged to develop and refine measurement tools with clinical utility for detecting and monitoring hypersensitivity, which can continue to elucidate the complexity behind pain responses and mechanisms. Application of GCT based model can assist the better clinical practice in chronic pain rehabilitation as well as improve research outcomes with the chronic pain population.
LIST OF ABBREVIATIONS
| IASP | = International Association for the Study of Pain |
| GCT | = Gate Control Theory |
| SG | = Substantia Gelatinosa |
| T-Cellfirst | = Central Transmission Cell |
AUTHORS' CONTRIBUTION
All authors contributed in the Conceptualization of the study, Writing - original draft.
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
This study was funded by the McMaster University School of Rehabilitation Science Graduate Scholarship, Canadian National Graduate Scholarship in Rehabilitation Science, CIHR Chair award (Gender in Measurement and Rehabilitation of Musculoskeletal Work Disability) and the Michael G. DeGroote Institute for Pain Research and Care.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.


