All published articles of this journal are available on ScienceDirect.
Efficacy and Safety of Phyllanthus Amarus Cream Treatment in Knee Osteoarthritis
Abstract
Background:
Knee osteoarthritis (OA) is a chronic degenerative joint disease and inflammatory pain which decreases daily activities.
Objective:
The aim of the present investigation was to examine skin permeation and skin irritation test of Phyllanthus Amarus (PA) cream including the effects of four weeks of treatment with PA cream in patients with osteoarthritis (OA) of the knee.
Methods:
The permeation study of PA cream was determined by Franz diffusion cells using a stillborn piglet skin. The primary irritation test was evaluated in rabbits and human volunteers following the Draize test. The study included sixty respondents diagnosed with symptomatic knee OA (12 males, 48 females). All the respondents volunteered to participate and randomly allocated into 3 groups including (n =20 in each group), followed by the placebo group and Phyllanthus Amarus (PA) cream group and NSAIDs cream group. They used a cream twice per day for four weeks. The patients were tested on 3 occasions; before two weeks and four weeks for the treatment period. The respondents were completely assessed for pain and function assessment by the visual analog scale (VAS) and the Western Ontario and McMaster Universities O-osteoarthritis Index questionnaire (WOMAC), respectively.
Results:
The PA cream showed good skin permeation after 10 hours. It had a high accumulative amount in the dermis and the receiving chamber more than the stratum corneum. Therefore, it could help to relieve pain and prolong the effect. The PA cream did not irritate the skin of rabbits and human volunteers. It is safe to be used in clinical treatment. The VAS and total WOMAC scores significantly decreased after 2 weeks (P < 0.001) and 4 weeks (P < 0.001) of intervention compared with before treatment in both the PA cream and NSAIDs groups in OA knee. However, the VAS and total WOMAC score of PA cream were not significantly compared with the NSAIDs groups.
Conclusion:
The Phyllanthus amarus cream is a new choice, and effective method for OA of the knee treatment. These data indicate that the treatment through Phyllanthus amarus cream improves pain relief and function.
1. INTRODUCTION
Knee osteoarthritis (OA) is a chronic degenerative joint disease which was caused by changing in bone joint structure with decreased hyaline articular joint, stiffness and progressive joint destruction. The primary OA knee symptoms are inflammatory pain and decreased daily activities and cause of disability in the elderly [1]. The treatment for OA knee is aimed at improving or maintaining function and symptoms. Generally, OA knee treatment are pharmacology [2] and joint replacement surgery is applied in severe patients. However, side effects of oral medications (acetaminophen and non-steroidal anti-inflammatory drugs (NSAIDs) for treating pain and rheumatic conditions, are irritation and damage the upper and lower gastrointestinal (GI) system [3]. Many types of local therapy cream for relieving symptomatic knee osteoarthritis (OA) treatment are NSAIDs cream like diclofenac and ketoprofen and herbal therapies cream/gel [4].
Phyllanthus Amarus is a traditional Thai herbal plant. It presents antioxidant capacity as the previous studies showed that it has bioactive compounds including polyphenols, gallic acid and flavonoids [5]. Putakala et al. [6] found that Phyllanthus Amarus was cardioprotective due to increased non-enzymatic and enzymatic antioxidants in the heart and aorta induced by high fructose in the rat. This plant has anti-inflammatory properties [7]. Harikrishnan et al. [8] found that Phyllanthus Amarus inhibited the inflammatory process via NF-κB/MAPK/PI3K-Akt signaling pathways in U937 macrophages. Moreover, it is suggested as a medicinal plant for treating hepatitis and anticancer activities [9].
However, the effectiveness of Phyllanthus Amarus cream has not been assessed. The underlying biological release and permeation processes in these subjects remain unknown. Thus, the purpose of this study was to determine the efficacy of the Phyllanthus Amarus cream treatment for knee OA.
2. MATERIALS AND METHODS
2.1. Skin Permeation Study
The amount of Phyllanthus Amarus cream permeated into the skin was evaluated by Franz diffusion cells [10]. The stillborn piglet skin was cleaned and hairs and subcutaneous fat tissue were removed. The skin was mounted between the donor chamber and the receiver chamber. Phosphate buffer (pH 5.5) was used as a receiver medium, which was constantly stirred at 100 rpm with a magnetic bar. The temperature of the receiver chamber was controlled at 32 ± 2°C. One gram of PA cream was added to the donor chamber. At the time of the intervals (30 min, 1, 2, 4, 6, 8 and 10 h), the receiver medium was withdrawn from the receiver chamber and replaced with an equal volume of the fresh medium. The samples were then analyzed by high-performance liquid chromatography (HPLC). The HPLC analysis was used [C-18 column (250 × 4.6mm i.d., 5 μm, Mightysil)] as a stationary phase. The mobile phase was composed of acetonitrile and 0.3% glacial acetic acid (15:85, pH 3) with a flow rate of 1.0 ml/min at room temperature. The injection volume was ten microliters and the detection wavelength was 280 nm. The reference standard was gallic acid. At the end of the experiment, each stillborn pig skin was washed with PBS. The amount of gallic acid in the stratum corneum (SC) were determined. The skin was stripped with 20 pieces of an adhesive tape. Each tape was charged with a weight (300 g) for 10 sec. The first tape strip was discarded. The 2nd to 20th tape strips were extracted with methanol and analyzed by HPLC. The amount of gallic acid in the viable epidermis and dermis (VED) was determined. The skin was cut into small pieces and extracted with methanol. The filtrate was then analyzed by HPLC. The experiments were done in triplicate. Gallic acid was used as a reference standard. The percentage of cumulative amount of gallic acid (%) in SC, VED and receiving solution was finally calculated using the following equation:
% cumulative amount of gallic acid = (Αsc, Αved, Αso ÷ Αtotal)×100 Asc, Aved and Aso are the amounts of gallic acid found in SC, VED and solution, respectively. Atotal is the amount of gallic acid in 1 g of formulation.
2.1.1. Skin Irritation Test in Rabbit
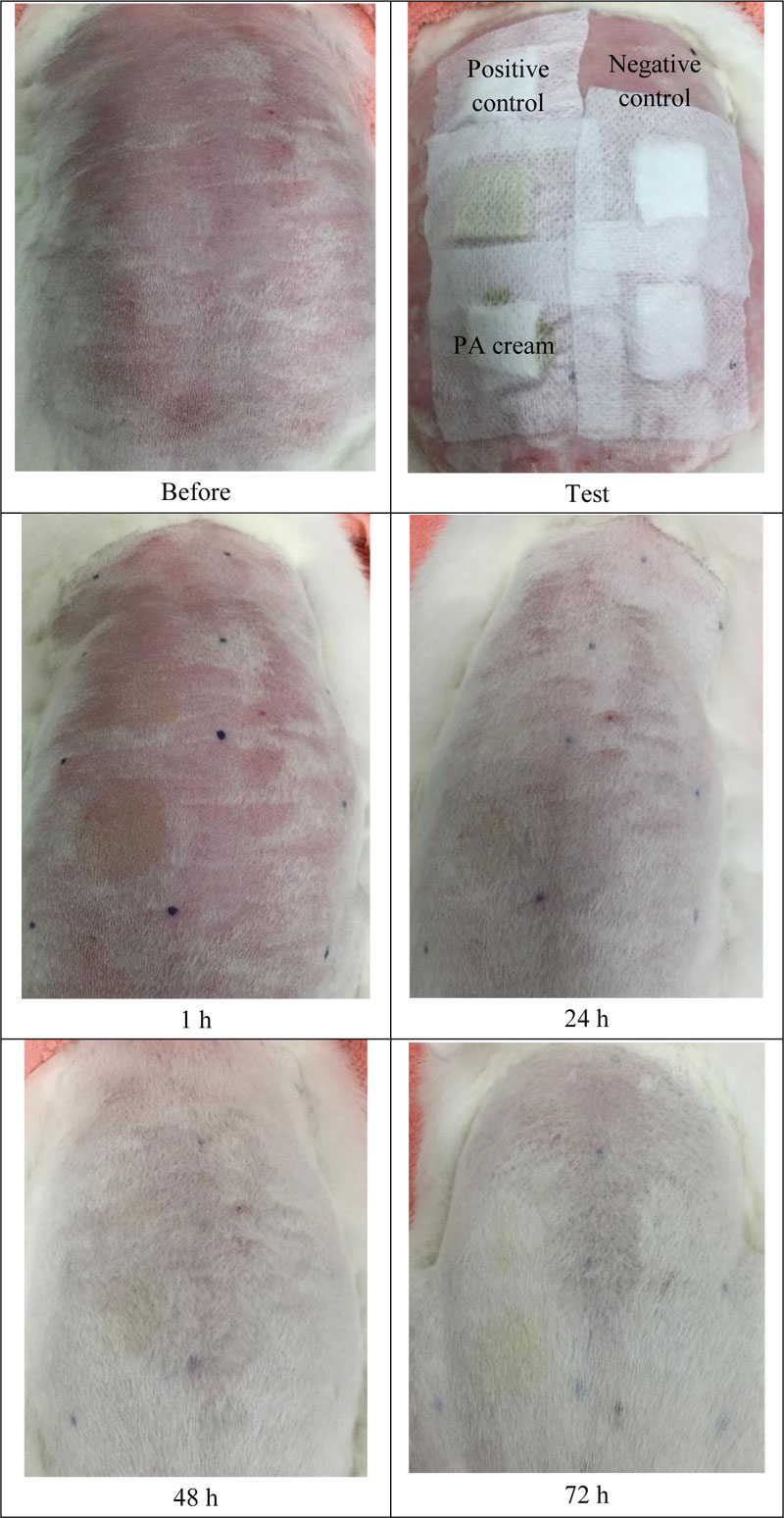
Acute skin irritation was evaluated using three male New Zealand white rabbits based on the Draize model [11]. For this section, the protocol was approved by the Committee on Research Ethics related to Animals, Experimentation of Laboratory animal center, Chiang Mai University. Fur was removed from the dorsal area of the trunk of the rabbit, approximately 24 h before the test. In this study, 2% (w/v) of sodium lauryl sulfate (SLS) was used as a positive control and untreated skin was used as the negative control. Then, 1.5 ml of each formulation was applied to 2 x 2 cm gauze patches and wrapped with 5 cm × 5 cm occlusive dressing on the rabbit trunk. At the end of the exposure period (4 h), the gauze patches were removed and the skin was cleaned with water. Skin irritation was observed and graded as erythema and edema reactions after patch removal for 1, 24, 48 and 72 h. The reactions were scored based on the Draize scoring system. The Primary Dermal Irritation Index (PII) calculated the following equation:
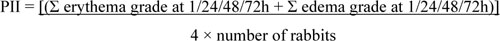 |
The irritation degree was classified based on the PDII values as non-irritation (PDII= 0-0.5), slight irritation (PDII= 0.5-2.0), moderate irritation (PDII= 2.1-5.0) or severe irritation (PDII= 5.0-8.0).
2.1.2. Skin Irritation Test in Human Volunteers
The skin irritation study was carried out by a patch test using the Finn chamber® in thirty healthy human volunteers aged between 25-60 years. All the volunteers did not have any skin disease, pharmacological treatment, skin atrophy, and tattoo. For this experiment, the protocol was approved by the Committee on Research Ethics of Faculty of Associated Medical Sciences, Chiang Mai University. The upper back of each volunteer was cleaned with water before the experiment. In this study, 2% (w/v) of SLS was used as a positive control, whereas untreated skin was used as a negative control. The test samples were added into the Finn chamber® and stuck on the upper back of volunteers for 48 h. The erythema and edema formation were observed at 1, 24, 48 and 72 h after patch removal. The average score of erythema and edema formation was represented according to the Draize scoring system. The primary dermal irritation index (PII) was calculated with the same equation mentioned in the skin irritation test in the rabbit protocol.
2.2. Participants
Sixty patients were diagnosed with OA knee followed the American College of Rheumatology (ACR) Classification Criteria [12]. The sample size required for each group was 15 [and add 15% for drop out] (power of 0.9 and significant level =0.05,) which was calculated from a previous study [13]. We included 20 patients in each group.This study was a randomized controlled clinical trial (RCT). Patients who were assessed for eligibility followed the inclusion criteria which were randomly assigned to one of the three treatment groups including placebo cream, Phyllanthus Amarus (PA) cream and NSAIDs cream. The inclusion criteria included subjects who had chronic knee pain for more than 3 months, aged between 50 and 80 years, morning stiffness < 30 min, and crepitus on the motion.The exclusion criteria included a history of osteoarthritis due to other causes such as infection in the knee joint, previous knee fractures or major trauma or analgesic drugs (opioids), injection of steroid within 3 months, or surgical knee replacement using prosthesis [14]. The study protocol was approved by the institutional review board of the Faculty of Associated Medical Sciences, Chiang Mai University. A written informed consent was taken from all the participants.
2.3. Treatment Procedures
The Phyllanthus Amarus cream and placebo cream were prepared by the pharmacologist. Patients of all groups used the cream twice per day for four weeks. The participants were randomized in the groups using a computer program and examined by the physician with double-blind to group assignment.
2.4. Outcome Measures
The clinical outcomes were evaluated before the intervention, two weeks, and four weeks after treatment.
| Test substances | Primary irritation index (PII) | Classification of skin reaction |
|---|---|---|
| PA cream | 0 | Non-irritation |
| Positive control | 0 | Slight irritation |
| Negative control | 1 | Non-irritation |
| Test substances | Primary irritation index (PII) | Classification of skin reaction |
|---|---|---|
| PA cream | 0.20 | Non-irritation |
| Positive control | 1.16 | Slightly irritation |
| Negative control | 0.09 | Non-irritation |
2.4.1. Visual Analogue Scale (VAS)
The severity of knee pain was assessed using the Visual Analog Scale (VAS) before and after treatment for two and four weeks. The scale of VAS is the standard numeric scale at 0 to 100. Zero (0) indicates the absence of pain and 100 indicates very severe pain/limitation.
2.4.2. WOMAC Score
The Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) score is widely assessed for the OA knee symptoms and the disability questionnaire. The disability questionnaire included 24 questions for patients with osteoarthritis. It consisted of self-reported knee pain, stiffness, and physical function. Subjects were assessed before treatment and two and four weeks after the start of the treatment.
2.5. Statistical Analysis
The values are expressed as mean ± SD. The data before treatment and after two weeks and four weeks of treatments were compared within the groups using the nonparametric (Wilcoxon) tests. Statistical analysis was carried out by repeated measures of ANOVA and was followed by Turkey’s post hoc test. p values of less than 0.05 were considered statistically significant.
3. RESULTS
3.1. Skin Permeation Study
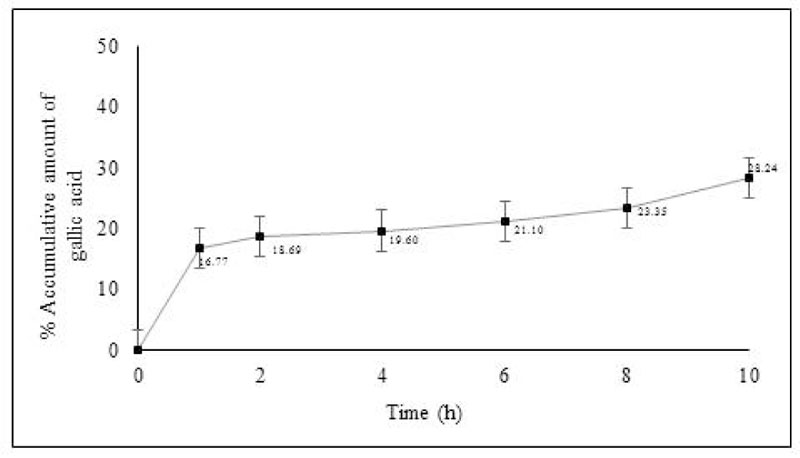
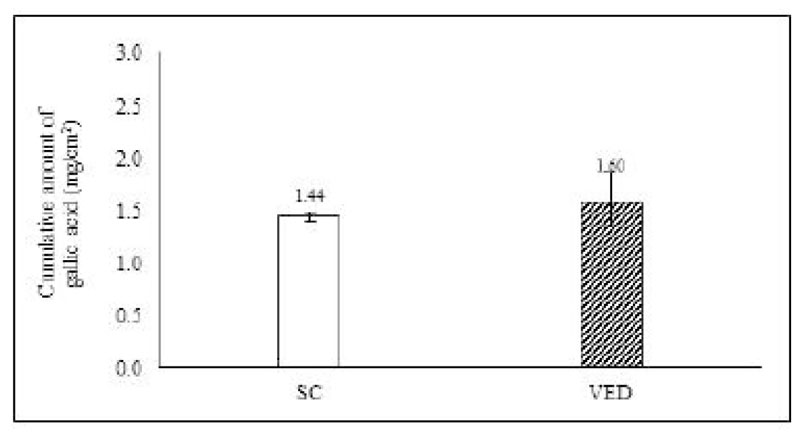
Gallic acid in the Phyllanthus Amarus (PA) cream was detected as a marker in skin permeation study. The amount of gallic acid penetration in stillborn piglet skin was studied by Franz diffusion cells. The percentage of the accumulative amount of gallic acid after 10 hours is shown in Fig. (1). The result showed that the PA cream could release and permeate into the receiving chamber within 1 hour. The PA cream continuously released till 10 hours and exhibited percentage of the accumulative amount of gallic acid after 10 hours as 28.24 ± 0.22. The skin was extracted after the end of the permeation study. The results showed that gallic acid from PA cream was also found in the stratum corneum (SC) and viable epidermis and dermis (VED). The cumulative amount of gallic acid in SC and VED is shown in Fig. (2). The PA cream showed its cumulative amount after 10 hours as 1.44 ± 0.04 mg/cm2 and 1.60 ± 0.26 mg/cm2 in SC and VED.
Skin irritation test in rabbits and human volunteers:
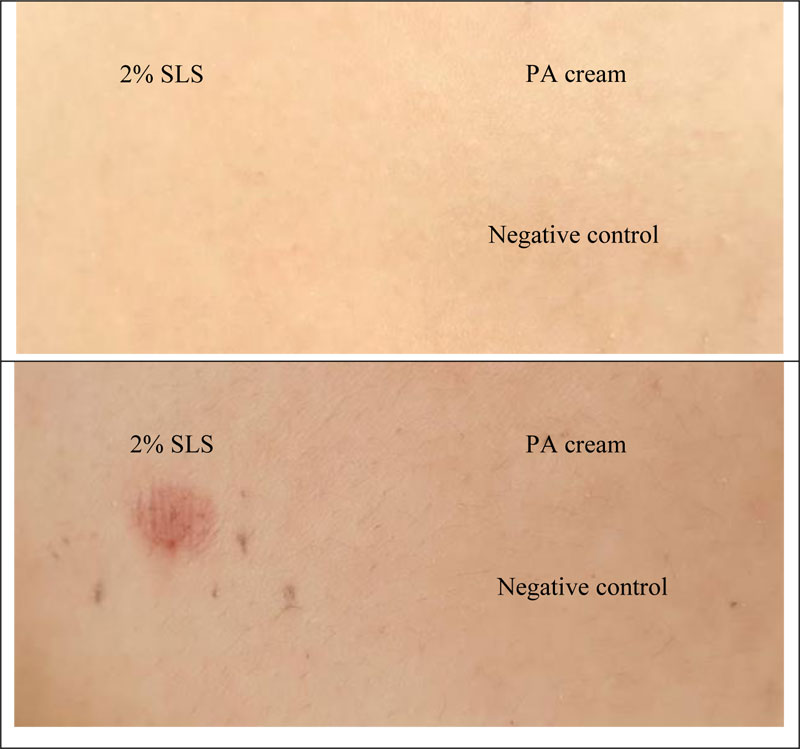
The skin irritation test is used to determine the safety of the PA cream. Accordingly, the erythema and the edema scores were recorded and calculated in terms of PDII. The data are shown in Table 1 and Fig. (3). The PA cream revealed non-irritation, whereas 2 w/v SLS (positive control) showed slight irritation.
Finn Chambers® occlusive patch test was used to study the skin irritation in 30 healthy human volunteers. The results are shown in Table 2 and Fig. (4). It was found that PA cream also exhibited no-skin irritation according to the result of the rabbit skin irritation test. Sodium lauryl sulfate (SLS) showed slight irritation. Therefore, the PA cream is safe for further use in clinical treatment.
3.2. Sample Description
Sixty patients with knee osteoarthritis were randomly allocated to placebo cream, Phyllanthus Amarus cream (PA cream) and NSAIDs cream groups. The mean ages in placebo cream, Phyllanthus Amarus cream and NSAIDs cream groups were 61.95 ± 6.47, 65.40 ± 6.75 and 61.1 ± 6.24 years, respectively. These three groups were similar to the characteristic findings and are presented in Table 3.
| Characteristics |
placebo cream (N=20) Mean ± SD |
Phyllanthus Amarus cream (n=20) Mean ± SD |
NSAIDs cream (n=20) Mean ± SD |
|---|---|---|---|
| Age (years) | 61.95± 6.47 | 65.40 ± 6.75 | 61.1±6.24 |
|
Gender Male (n) Female (n) |
4 16 |
3 17 |
5 15 |
| BMI (kg/m2) | 24.27 ± 2.76 | 24.72± 5.14 | 24.72± 5.14 |
| Disease duration (years) | 2.00 ± 1.30 | 2.00 ± 1.60 | 2.23± 1.14 |
Data are expressed as mean ± standard deviation
|
Weeks Parameters |
placebo cream (N=20) Mean ± SD |
Phyllanthus Amarus cream (n=20) Mean ± SD |
NSAIDs cream (n=20) Mean ± SD |
||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 2 | 4 | 0 | 2 | 4 | 0 | 2 | 4 | |
| VAS | 66.10± 9.71 |
64.05± 8.73 |
63.05± 8.69 |
65.30 ± 10.67 |
46.20± 9.06* |
25.75± 10.04* |
67.5± 9.81 |
50± 6.88*,# |
29.95± 7.69*,# |
| WOMAC | 94.55 ± 20.43 |
90.60± 18.87 |
88.2± 21.59 |
100± 18.73 |
64.45± 9.44* |
35.40± 15.09* |
89.75± 23.83 |
69.60± 15.74*,# |
36.7± 11.40*,# |
* showed significant pretreatment in each group, # showed significant post-treatment wered to a period of 2 weeks in each group, p < 0.001. Data are expressed as mean ± standard deviation.
3.3. The Effect of Phyllanthus Amarus Cream on the Visual Analog Scale (VAS)
Pre-treatment of all groups was not significant (p < 0.05). Post-treatment after 4 weeks, showed that the pain score was reduced significantly compared with post-treatment after 2 weeks (p < 0.001). The Phyllanthus Amarus cream and NSAIDs cream had significantly reduced VAS score compared to a placebo cream group after two weeks (p < 0.05), and four weeks (p < 0.001), of treatment (Table 4). However, the effect on VAS score of PA cream and NSAIDs cream for pre and post-treatment two weeks and four treatments were not significant (p < 0.05). This data suggested that the PA cream group reduced knee pain.
3.4. The Effect of Phyllanthus Amarus Cream on Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC)
The total WOMAC of pre-treatment of three groups; placebo, PA and NSAIDs cream was 94.55± 20.43, 100±18.73, and 89.75±23.83, respectively as Table 4. Both PA and NSAIDs cream groups after treatment for 2 and 4 weeks were significantly decreased. The total WOMAC score (p < 0.001) was compared to pre-treatment. Two and four weeks for the treatment of PA cream were not significantly compared to NSAIDs cream group (p < 0.05). The study findings suggested that PA cream reduced the total WOMAC score. There was a significant decrease in total WOMAC in both groups (p < 0.001) (Table 4). The PP group showed significantly more total WOMAC than the US group.
4. DISCUSSION
In this study, we found that Phyllanthus Amarus cream had an acceptable efficacy compared to the NSAIDs cream for relieving pain and improving the function of patients with knee OA. It was effective in for relieving pain. Phyllanthus Amarus cream showed good skin penetration within 10 hours. Moreover, it could penetrate deep into the dermis and blood circulation than stratum corneum. Therefore, it reduced pain and prolongs treatment. The phyllantus cream had anti–inflammation and anti-oxidants including polyphenols, gallic acid, and flavonoids [5] that help to relieve pain via many in vitro and in vivo with several mechanisms [15-17] including NF-κB/MAPK/PI3K-Akt signaling [8]. This study showed that Phyllanthus Amarus cream significantly reduced pain pretreatment, while there was no significant effect compared to NSAIDs cream after treatment. There were some studies according to the current study. Trnavský et al. [18] found that 5% of ibuprofen cream treatment is safe and reduced pain according to the visual analog scale (VAS) score compared to a placebo cream. An acetylated fatty acid topical cream application twice per day for 30 days of treatment reduced pain [19]. Moreover, after treatment, PA cream showed improvement of knee function by WOMAC assessment according to the study of Cohen et al. [20] This study found that glucosamine and chondroitin sulfate improved WOMAC within 4 weeks and also reduced pain. The efficacy of PA cream and NSAIDs cream was not significant after treatment for 2 and 4 weeks. They also relieved pain and improved function assessment. Many researchers showed the topical cream including transdermal bioidentical progesterone cream as hormonal treatment of OA has a role in regulating cartilage and bone remodeling [21], a cream containing glucosamine sulfate, chondroitin sulfate, and camphor, topical nimesulide and capcicin cream [4]. However, the research showed that the topically applied cream with 10% trolamine salicylate did not relieve pain compared to the placebo cream in OA knee [22]. Moreover, topical herbal therapies such as the application of Arnica montana fresh plant gel improved pain symptoms and function in mild to moderate OA of the knee at twice daily applied twice daily [23]. Besides, the use of Phyllanthus Amarus plant to produce a cream, it is also used for treating several diseases such as jaundice, diabetes, stomach-ache, hepatitis-C. It also has pharmacological activities such as antiviral, antibacterial, anticancer, antimalarial, antimicrobial, antidiabetic, hepatoprotective and nephroprotective [24]. However, further research should be studied at a younger age in various conditions including muscle pain. This study showed the PA cream is safe, with no irritation and effective treatment of OA of the knee.
CONCLUSION
Phyllanthus Amarus cream was used for relieving pain and improving WOMAC in OA knee. It showed good skin penetration and prolong releasing after 10 hours improve pain treatment. Furthermore, our findings suggested that Phyllanthus Amarus cream is a safe and effective treatment for improving knee function in knee OA patients.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study was approved by the institutional review board of the Faculty of Associated Medical Sciences, Chiang Mai University (number AMSEC-61FB-005) and laboratory animals at laboratory animal center, Chiang Mai University (number 2562/RB-0002), Thailand.
HUMAN AND ANIMAL RIGHTS
All the reported experiments on humans were in accordance with the ethical standards of the committee responsible for human experimentation (institutional national), and with the Helsinki Declaration of 1975, as revised in 2008 (http://www.wma.net/en/20activities/10ethics/10helsinki/).
All the reported experiments on animals were in accordance with the ethical standards of the review board of the Faculty of Associated Medical Sciences, Chiang Mai University, China.
CONSENT FOR PUBLICATION
All the participants were informed about the protocol and gave their written informed consent before participating in the study.
AVAILABILITY OF DATA AND MATERIALS
The data and materials that support the findings of this research are available from the corresponding author upon reasonable request.
FUNDING
This work was supported by the grants funded by Agricultural Research Development Agency (Public Organization) or ARDA, Thailand.
CONFLICT OF INTEREST
The author declares no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.


