All published articles of this journal are available on ScienceDirect.
Does The Menstrual Cycle Phase Influence Physical Fitness Performance In Athletes And Non-athlete Female University Students With Mild Menstrual-Related Symptoms?
Abstract
Introduction
Previous studies have explored the influence of the menstrual cycle on various aspects of exercise performance. However, associations between menstrual cycle phases and fitness performance in women remain inconclusive. This study aimed to examine the effects of the menstrual cycle and premenstrual symptoms on physical fitness test outcomes among female university students with and without regular exercise habits.
Methods
Eighteen female athletes and 13 female non-athlete university students volunteered for this study. Only those who completed all test sessions were included in the final analysis, resulting in the analysis of data from eight athletes (height: 1.66 ± 0.04 m; weight: 57.1 ± 2.9 kg) and eight non-athletes (height: 1.62 ± 0.05 m; weight: 58.0 ± 6.7 kg). Basal body temperature was used to categorize the menstrual cycle into three phases: menstrual, follicular, and luteal. A validated questionnaire assessed physical and mental symptoms related to menstruation. Fitness tests included handgrip strength, number of sit-ups, sit-and-reach test, number of side-steps, 1000-m run, 50-m sprint, and standing long jump.
Results
In both groups, physical symptoms differed significantly between the menstrual phase and other phases. Mental symptoms showed no significant changes in athletes, whereas three mental symptoms varied significantly in non-athletes. No significant interaction was observed between the group and menstrual cycle phase in any physical fitness measure. Athletes outperformed non-athletes in all tests, except for handgrip strength, regardless of the menstrual cycle phase.
Discussion
These findings suggest that among women with mild premenstrual symptoms, the menstrual cycle may have a limited impact on physical fitness, regardless of training status. These outcomes might be relevant for physical fitness professionals and researchers, improving the understanding of the effects of menstrual cycle phases on physical fitness and psychological symptoms in women.
Conclusion
The menstrual cycle appears to have a minimal impact on physical fitness test performance among female university students with mild menstrual symptoms, regardless of training status.
1. INTRODUCTION
The menstrual cycle (Fig. 1) is a unique physiological process in females, characterized by cyclical hormonal fluctuations and changes in body temperature that can influence both physical and psychological states [1, 2]. Menstruation typically begins around 13 years old and continues for approximately 40 years, excluding periods of pregnancy or hormonal contraception use [3]. Menstrual symptoms—encompassing both physical and mental complaints—are commonly categorized into conditions such as dysmenorrhea, menorrhagia, and premenstrual syndrome (PMS) [4]. Among these, PMS is particularly prevalent among women of reproductive age and is characterized by emotional, physical, and behavioral symptoms, particularly during the late luteal phase [5].
Regular physical exercise has been recommended as a non-pharmacological intervention for PMS [6], with several studies [7-12] suggesting that such activity may alleviate symptoms that negatively affect daily activities, including academic performance and school attendance. However, the findings remain inconsistent and the current literature lacks a sufficient number of high-quality randomized controlled trials to draw definitive conclusions [13]. From the perspective of athletic performance, while the menstrual cycle is frequently considered a potential influence on physical condition and competitive output, even among elite athletes, research has not reached a consensus. Female athletes are increasingly recognized for their ability to maintain optimal performance regardless of their menstrual phase, emphasizing the need for individualized management strategies. Despite numerous studies examining relationships between menstrual cycle phases and physical performance [14-17], results remain inconclusive and no consistent patterns have emerged. For instance, the premenstrual phase is often associated with poorer physical and mental conditions [18], the ovulation and luteal phases have been considered to show increased knee joint laxity [19], and the luteal phase is commonly believed to impair endurance performance [20, 21], but some athletes have demonstrated peak performance even during these phases. Such findings highlight the presence of substantial inter-individual variability and support the growing perspective that menstrual-related responses should be addressed through a personalized framework.
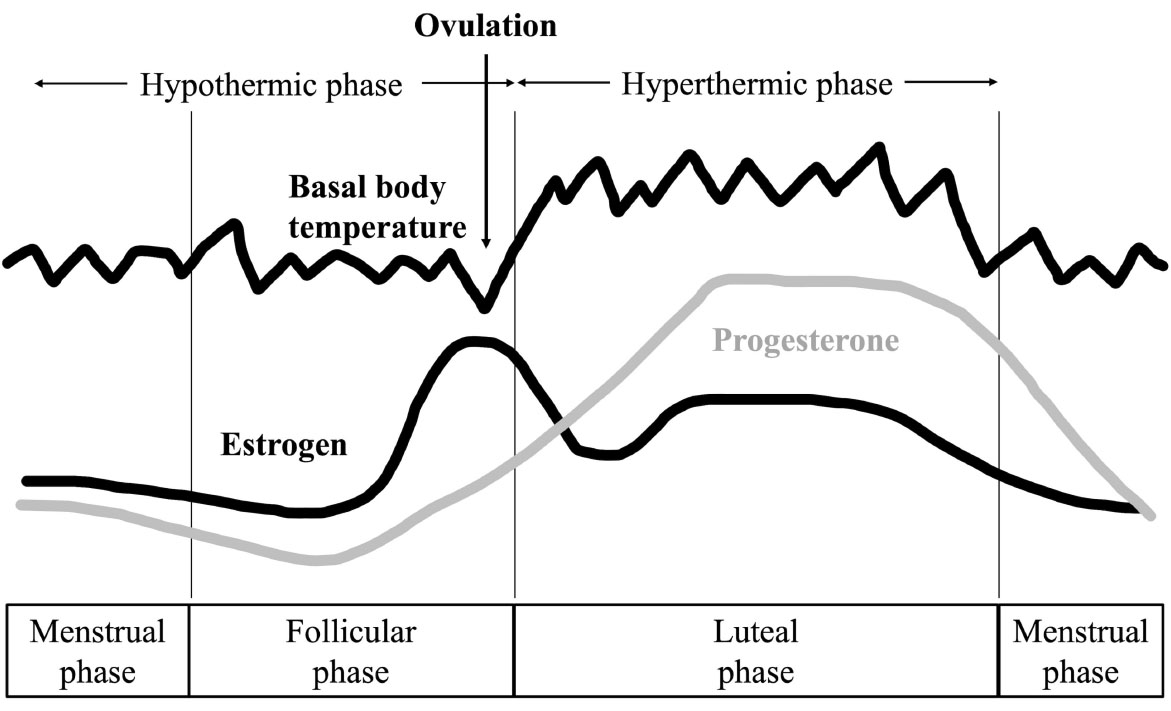
Changes in basal body temperature and hormones associated with menstruation.
Research also suggests that the impacts of menstrual cycle-related hormonal changes on physiological functions, such as thermoregulation, may differ between athletes and non-athletes. For example, Kuwahara et al. [22] reported that long-term physical training enhanced heat dissipation during the luteal phase. This raises the possibility that hormone-induced variations in performance metrics—such as muscular strength [23], flexibility [24], and agility [25, 26]—may manifest differently depending on training status. Although PMS is a common experience among athletes and non-athletes, the severity and effects may vary according to physical activity levels [27]. These observations underscore the need for further empirical investigation into how menstrual cycle dynamics interact with various components of physical fitness across different populations. In this context, hormonal fluctuations throughout the menstrual cycle are known to exert broad physiological and psychological effects [3-5, 14-17]. Although multiple aspects of physical performance may be influenced by these changes, the extent to which these effects differ between athletes and non-athletes remains unclear. To the best of our knowledge, this is the first study to simultaneously examine menstrual symptoms and multiple components of physical fitness as measured by the national standardized test in Japan while directly comparing athletes and non-athletes through integrated assessment.
Based on these considerations, we hypothesized that the influence of the menstrual cycle on fitness performance varies depending on exercise habits. This study, therefore, aimed to examine the relationship between menstrual-related symptoms and physical fitness outcomes across different menstrual phases, classified using basal body temperature (BBT), among both trained and untrained female university students. Although BBT offers relatively limited accuracy for phase classification, it is noninvasive and easily measured using commercially available thermometers, thereby increasing accessibility and facilitating replication. What distinguishes this study from previous research is the integrated evaluation of menstrual symptoms and physical fitness using a comprehensive fitness test protocol, combined with standardized procedures and an accessible method for phase classification.
2. MATERIALS AND METHODS
2.1. Participants
A total of 18 female university athletes and 13 non-athlete female students voluntarily participated in this study. All participants completed a pre-screening questionnaire to assess eligibility. The inclusion criteria were as follows: 1) a self-reported regular menstrual cycle of 25–38 days, with a typical menstrual duration of 3–7 days; 2) a biphasic BBT pattern indicating ovulatory cycles; 3) absence of any injury or illness that could interfere with physical activity; 4) for athlete participants, current participation in at least one university athletic club; and 5) for non-athlete participants, classification as physically inactive according to Ministry of Health, Labour and Welfare guidelines (i.e., not engaging in exercise for ≥30 min at least twice per week over the past year). Only participants who successfully completed all test sessions were included in the final analysis, resulting in a sample of eight athletes (mean age: 20.3 ± 1.4 years; height: 1.66 ± 0.04 m; weight: 57.1 ± 2.9 kg) and eight non-athletes (mean age: 21.1 ± 2.5 years; height: 1.62 ± 0.05 m; weight: 58.0 ± 6.7 kg). During the study, a total of 15 participants withdrew (mainly due to injury or illness), leading to a reduced cohort available for analysis. All participants provided written informed consent after receiving a detailed explanation of the study procedures and potential risks. The study protocol was approved by the Research Ethics Committee of Tokyo Gakugei University (approval no. 636) and was conducted in accordance with the Declaration of Helsinki.
2.2. Classification of Menstrual Cycle Phases and Data Collection Timing
Menstrual cycle phases were determined using BBT, a non-invasive method for estimating hormonal fluctuations. While hormone assays such as blood or saliva testing are commonly used for precise phase classification [16], BBT is considered a valid alternative for women with regular ovulatory cycles. In addition, some reports on women with normal menstruation have classified the menstrual cycle into two phases based on BBT and regular menstrual cycle, and examined mental and physical condition and performance [19, 27-30]. Given this background, the present study classified phases of the menstrual cycle based on BBT data.
Participants were informed verbally and in writing that BBT should be measured: 1) at the same time each morning; 2) while lying down after waking, without getting up; and 3) using actual values. Participants were then divided into periods based on the assumption that these conditions were met.
Participants recorded BBT daily using commercially available digital thermometers (MC-652LC; Omron, Kyoto, Japan or BT-14; Nishimoto, Mie, Japan or MT1622J; Morishita Jintan, Osaka, Japan). Measurements were taken while lying in bed immediately upon waking, ideally at the same time each morning, as mentioned above. BBT logs were submitted weekly via Google Forms to ensure compliance and completeness.
Following the method proposed by Kusuda and Onoue [31], Day 1 was defined as the first day of menstruation. Mean BBT for Days 1–10 was calculated, and a sustained increase of ≥0.1°C for at least three consecutive days above this average was used to define the transition to the high-temperature (luteal) phase. Based on this classification and previous studies [19, 29], measurements were taken once for each of the following periods: the menstrual phase, when bleeding was confirmed, from the start of menstruation until day 7; the follicular phase, from day 4 to day 7 after the end of menstruation; and the luteal phase, from day 3 to day 10 of the high-temperature phase.
2.3. Measurements
2.3.1. Menstrual-related Symptoms
Menstrual-related symptoms were assessed using a questionnaire adapted from the Menstrual Distress Questionnaire (MDQ) originally developed by Moos [32]. The questionnaire was administered on the same day as the fitness test for each of the menstrual phases. The present study employed a questionnaire developed by Hayamizu [29], which was created with reference to Hashimoto’s Female Athlete Condition Survey [33] and Moos’s MDQ Form T [34]. Hayamizu’s questionnaire consists of 18 physical and 12 mental symptoms, selected by researchers with experience as female athletes and/or in coaching female athletes, including the first author of the present study. With approximately two-thirds the number of items compared to the original versions, this questionnaire was designed to reduce the response burden while retaining relevance to athletic contexts. Each item was rated using a four-point Likert scale: 0, no symptoms; 1, mild symptoms with no interference in daily life; 2, moderate symptoms with occasional interference in daily activities; and 3, severe symptoms.
2.3.2. Physical Fitness Test
Physical fitness was assessed using the standardized fitness test protocol established by the Japan Sports Agency [35], which evaluates various components of physical fitness. The following seven test items were included: handgrip strength, number of sit-ups, sit-and-reach test, side-step test, 1000-m endurance run, 50-m sprint, and standing long jump.
Handgrip strength was measured using a dynamometer with the grip span set to a 90° angle at the second knuckle of the index finger. Participants stood upright with arms relaxed and feet naturally apart, holding the device without it touching the body or clothing. Each hand was tested twice alternately, and the mean of the highest values from each hand was used for analysis.
The sit-up test was performed in a supine position on a mat with knees bent to 90°. Arms were crossed over the chest, and an assistant stabilized the legs. Participants raised their upper body until both elbows touched their thighs, repeating the movement as many times as possible in 30 seconds. Repetitions were excluded if the back did not fully contact the mat between sit-ups. The final score was the number of valid repetitions in 30 seconds.
The sit-and-reach test was performed in a long-sitting position with the back and buttocks against a wall. Hands were placed palm-down, shoulder-width apart, on the measuring box. After pushing on the box to extend the back while keeping the elbows straight and chest upright, the participant reached forward without lifting their hands from the box. The score was the distance the box moved from the start to the point of maximal reach. Two trials were performed, and the better result was used for analysis.
In the side-step test, the participant straddled the center line of three parallel lines spaced 100 cm apart. For 20 seconds, the participant moved laterally at maximal effort, stepping on or over each outer line with the respective foot. One point was given per line crossed, and the higher of the two trial scores was used for analysis.
Both the 1,000-m run and the 50-m sprint were performed once at maximal effort. The 1,000-m run was conducted with a standing start, and the 50-m sprint with a crouching start. Completion times were recorded for each test.
The standing long jump was performed from a natural stance with feet parallel and toes at the takeoff line. Participants jumped forward with a simultaneous two-foot takeoff. The distance from the takeoff line to the nearest heel on landing was measured. Two trials were conducted, with the better result used for analysis.
All measurements were conducted on a single day. The order of test items was randomized for each participant, except for the 1000-m run, which was consistently administered last in accordance with the Japan Sports Agency guidelines [35]. To minimize the impact of test-order fatigue, a 5- to 10-minute recovery interval was provided between each test. In addition, before proceeding to the next test, verbal confirmation of physical readiness was obtained from each participant. The recovery duration was determined with reference to studies on phosphocreatine resynthesis after short-term, high-intensity exercise, which is related to several of the fitness tests conducted in this study [36, 37], as well as a study employing the same side-step test as in the present study [26]. Taken together, these measures suggest that the influence of test order on fatigue was unlikely to have affected our results.
Participants completed a standardized warm-up for approximately 15 minutes that included a 400-m jog, stretching exercises, and movement drills (e.g., high knees, butt kicks, and skipping for 30 m, repeated twice). A 30-m sprint was performed at the end of the warm-up. Body weight was measured immediately prior to the warm-up using a digital scale (HBF-254C; Omron).
2.4. Statistical Analysis
Fitness test results are expressed as the mean (standard deviations). Body weight and MDQ scores are reported as medians. A two-way repeated-measures analysis of variance (ANOVA) was used to assess the effects of group (athlete vs. non-athlete) and menstrual phase on fitness test performance. When significant main effects or interactions were found, Bonferroni-adjusted post hoc tests were performed. For non-parametric variables (i.e., body weight and MDQ scores), Friedman’s test was used. Effect sizes for ANOVA were estimated using partial eta squared (η2), interpreted as small (0.01), medium (0.06), or large (0.14). For parametric comparisons, Cohen’s d was calculated and interpreted as small (0.2), medium (0.5), or large (>0.8), as described elsewhere [38]. Effect sizes for non-parametric comparisons are expressed as r and interpreted as small (r = 0.10), medium (r = 0.30), or large (r = 0.50).
All analyses were conducted using SPSS Statistics for Windows (version 29; IBM, Chicago, IL, USA), and effect-size calculations were performed using Excel for Windows (version 2209; Microsoft, Seattle, WA, USA). The level of statistical significance was set at p < 0.05.
3. RESULTS
3.1. Body Weight and Menstrual-Related Symptoms
No significant differences in body weight were observed across menstrual phases in either athlete or non-athlete groups. Table 1 presents the results of the MDQ for physical and psychological symptoms in the athlete group, while Table 2 displays corresponding results for the non-athlete group. Among athletes, several physical symptoms showed significant variation across menstrual phases, including “lower abdominal pain”, “acne”, “skin irritation”, “fatigue”, and a subjective sense of “impaired physical coordination”. Post hoc analyses indicated that the rating for “impaired physical coordination” was significantly higher during the menstrual phase compared to other phases. However, no significant differences in psychological symptom scores were found within the athlete group.
| - | Luteal | Menstrual | Follicular | χ2(2) | - | p | r | Post Hoc |
|---|---|---|---|---|---|---|---|---|
| - | (median) | (median) | (median) | |||||
| Physical symptoms (n = 8) | - | - | - | - | - | - | - | - |
| 1. Abdominal pain | 0 | 0 | 0 | 6.00 | * | 0.05 | 2.12 | n.s. |
| 2. Abdominal bloating | 0.5 | 0.5 | 0 | 4.30 | - | 0.12 | 1.52 | n.s. |
| 3. Diarrhea | 0 | 0.5 | 0 | 2.00 | - | 0.37 | 0.71 | n.s. |
| 4. Constipation | 0 | 0 | 0 | 2.00 | - | 0.39 | 0.71 | n.s. |
| 5. Headache | 0 | 0.5 | 0 | 4.76 | - | 0.09 | 1.69 | n.s. |
| 6. Stiff shoulder | 0 | 0 | 0 | 2.00 | - | 0.37 | 1.71 | n.s. |
| 7. Nausea | 0 | 0 | 0 | 0.00 | - | 1.00 | 0.00 | n.s. |
| 8. Dizziness | 0 | 0 | 0 | 0.00 | - | 1.00 | 0.00 | n.s. |
| 9. Breast tenderness | 0 | 0 | 0 | 3.50 | - | 0.17 | 1.24 | n.s. |
| 10. Swelling of face and limbs | 0 | 0 | 0 | 5.60 | - | 0.06 | 1.98 | n.s. |
| 11. Drowsiness | 1.0 | 1.0 | 0 | 4.62 | - | 0.10 | 1.63 | n.s. |
| 12. Insomnia | 0 | 0 | 0 | 4.00 | - | 0.14 | 1.41 | n.s. |
| 13. Acne | 1.0 | 1.0 | 0 | 7.14 | * | 0.03 | 2.52 | n.s. |
| 14. Rough skin | 1.0 | 0 | 0 | 7.71 | * | 0,.02 | 2.52 | n.s. |
| 15. Food craving | 1.0 | 0.5 | 0 | 2.94 | - | 0.23 | 1.04 | n.s. |
| 16. Throat | 0 | 0 | 0 | 4.00 | - | 0.14 | 1.41 | n.s. |
| 17. Fatigue | 0 | 1.0 | 0 | 7.00 | * | 0.03 | 2.48 | n.s. |
| 18. Difficulties in coordination | 0 | 1.0 | 0 | 11.84 | ** | 0.00 | 4.19 | M> L, F |
| Mental symptoms (n = 8) | - | - | - | - | - | - | - | - |
| 1. Emotional outbursts | 0 | 0.5 | 0 | 3.87 | - | 0.14 | 1.37 | n.s. |
| 2. Irritability | 0 | 0 | 0 | 2.00 | - | 0.37 | 0.71 | n.s. |
| 3. Lack of concentration | 0 | 0.5 | 0 | 2.00 | - | 0.37 | 0.71 | n.s. |
| 4. Depression | 0 | 0 | 0 | 0.66 | - | 0.72 | 0.24 | n.s. |
| 5. Excitement | 0 | 0 | 0 | 2.00 | - | 0.37 | 0.71 | n.s. |
| 6. Anxiety | 0 | 0 | 0 | 2.00 | - | 0.37 | 0.71 | n.s. |
| 7. Apathy | 0 | 0 | 0 | 1.40 | - | 0.50 | 0.50 | n.s. |
| 8. Secluding oneself | 0 | 0 | 0 | 2.00 | - | 0.37 | 0.71 | n.s. |
| 9. Low capacity | 0 | 0 | 0 | 2.60 | - | 0.27 | 0.92 | n.s. |
| 10. Tearful | 0 | 0 | 0 | 2.00 | - | 0.37 | 0.71 | n.s. |
| 11. Mood swings | 0 | 0 | 0 | 2.60 | - | 0.27 | 0.92 | n.s. |
| 12. Feeling sick | 0 | 0 | 0 | 0.00 | - | 1.00 | 0.00 | n.s. |
| - | Luteal | Menstrual | Follicular | χ2(2) | - | p | r | Post Hoc |
|---|---|---|---|---|---|---|---|---|
| - | (median) | (median) | (median) | |||||
| Physical symptoms (n = 8) | - | - | - | - | - | - | - | - |
| 1. Abdominal pain | 0 | 0.5 | 0 | 4.76 | - | 0.09 | 1.69 | n.s. |
| 2. Abdominal bloating | 0 | 0 | 0 | 3.00 | - | 0.22 | 1.06 | n.s. |
| 3. Diarrhea | 0 | 0 | 0 | 2.00 | - | 0.37 | 0.71 | n.s. |
| 4. Constipation | 0 | 0 | 0 | 2.00 | - | 0.37 | 0.71 | n.s. |
| 5. Headache | 0 | 1.0 | 0 | 7.53 | * | 0.02 | 2.58 | n.s. |
| 6. Stiff shoulder | 0 | 0 | 0 | 3.00 | - | 0.22 | 1.06 | n.s. |
| 7. Nausea | 0 | 0 | 0 | 2.00 | - | 0.37 | 0.71 | n.s. |
| 8. Dizziness | 0 | 0 | 0 | 2.00 | - | 0.37 | 0.71 | n.s. |
| 9. Breast tenderness | 0 | 0.5 | 0 | 6.00 | * | 0.05 | 2.12 | n.s. |
| 10. Swelling of face and limbs | 0 | 0 | 0 | 0.20 | - | 0.91 | 0.07 | n.s. |
| 11. Drowsiness | 0.5 | 1.0 | 0 | 4.30 | - | 0.12 | 1.52 | n.s. |
| 12. Insomnia | 0 | 0 | 0 | 3.60 | - | 0.17 | 1.27 | n.s. |
| 13. Acne | 0 | 0 | 0 | 5.00 | - | 0.08 | 1.77 | n.s. |
| 14. Rough skin | 0 | 0 | 0 | 1.07 | - | 0.58 | 0.38 | n.s. |
| 15. Food craving | 0.5 | 0.5 | 0 | 5.64 | - | 0.06 | 1.99 | n.s. |
| 16. Throat | 0 | 0 | 0 | 0.80 | - | 0.67 | 0.28 | n.s. |
| 17. Fatigue | 0 | 1.0 | 0 | 1.60 | - | 0.45 | 0.59 | n.s. |
| 18. Difficulties in coordination | 0 | 0.5 | 0 | 2.00 | - | 0.37 | 0.71 | n.s. |
| Mental symptoms (n = 8) | - | - | - | - | - | - | - | - |
| 1. Emotional outbursts | 0 | 0 | 0 | 3.00 | - | 0.23 | 1.06 | n.s. |
| 2. Irritability | 0 | 0.5 | 0 | 8.00 | * | 0.02 | 2.83 | n.s. |
| 3. Lack of concentration | 0 | 1.0 | 0 | 1.50 | - | 0.47 | 0.54 | n.s. |
| 4. Depression | 0.5 | 1.0 | 0 | 3.50 | - | 0.17 | 1.24 | n.s. |
| 5. Excitement | 0 | 0 | 0 | 4.00 | - | 0.14 | 1.41 | n.s. |
| 6. Anxiety | 0 | 0.5 | 0 | 4.66 | - | 0.10 | 1.65 | n.s. |
| 7. Apathy | 0 | 1.0 | 0 | 6.42 | * | 0.04 | 2.27 | n.s. |
| 8. Secluding oneself | 0 | 0 | 0 | 2.46 | - | 0.29 | 0.87 | n.s. |
| 9. Low capacity | 0 | 0 | 0 | 2.66 | - | 0.26 | 0.94 | n.s. |
| 10. Tearful | 0 | 0.5 | 0 | 5.37 | - | 0.07 | 1.90 | n.s. |
| 11. Mood swings | 0 | 0.5 | 0 | 7.53 | * | 0.02 | 2.67 | n.s. |
| 12. Feeling sick | 0 | 0 | 0 | 2.00 | - | 0.37 | 0.71 | n.s. |
In contrast, the non-athlete group demonstrated significant phase-related differences in the physical symptoms of “headache” and “breast tenderness”, as well as in the psychological symptoms of “irritability”, “lack of motivation”, and “emotional instability”. Nonetheless, post hoc comparisons did not identify any significant differences between specific phases for these symptoms.
Individual data showed that the most severe level of menstrual-related symptoms—those significantly interfering with daily life—was observed in one non-athlete and two athletes. In the non-athlete group, one participant experienced “severe” symptoms during the luteal phase, including drowsiness and food cravings. During the menstrual phase, the same participant reported symptoms such as swelling of the face, limbs, and throat, excitement, anxiety, tearfulness, and mood swings.
Among athletes, two participants were identified as experiencing symptoms at the “severe” level. Athlete A reported stiff shoulders, drowsiness, and insomnia as physical symptoms during the luteal phase, and nearly all symptom items except for excitement in the mental symptom during the menstrual phase. That same athlete reported drowsiness, fatigue, feeling sick, difficulties in coordination, and depression. Athlete B reported the following symptoms during the menstrual phase: abdominal pain, diarrhea, breast tenderness or swelling of the face and limbs, food craving, anxiety, and tearfulness.
3.2. Physical Fitness Test
Figure 2a-g and Table 3 summarize the results of the physical fitness test. No significant interaction effects between group (athlete vs. non-athlete) and menstrual cycle phase were observed, nor were any significant main effects of menstrual phase alone identified. Effect sizes for performance changes across menstrual phases ranged from 0.00 to 0.80 in the athlete group and from 0.03 to 0.30 in the non-athlete group, indicating predominantly trivial to small effects. In contrast, significant main effects of group were observed across several test items. The athlete group performed significantly better than the non-athlete group in the number of sit-ups, sit-and-reach test, number of side-steps, and standing long jump, with large effect sizes (Cohen’s d = 1.90–5.90). Moreover, athletes recorded significantly faster times in both the 1000- and 50-m runs. No significant difference in handgrip strength was identified between groups.
| Test | Factor | df | F | P | - | ηp2 | Post Hoc Test |
|---|---|---|---|---|---|---|---|
| Handgrip strength | group | 1 | 2.86 | 0.11 | - | 0.17 | - |
| menstrual phase | 2 | 3.06 | 0.06 | - | 0.18 | - | |
| group × menstrual phase | 2 | 0.01 | 0.99 | - | 0.00 | - | |
| - | - | - | - | - | - | - | - |
| Sit-ups | group | 1 | 49.46 | 0.00 | ** | 0.78 | UT < T |
| menstrual phase | 2 | 1.99 | 0.15 | - | 0.12 | - | |
| group × menstrual phase | 2 | 1.65 | 0.21 | - | 0.11 | - | |
| - | - | - | - | - | - | - | - |
| Sit-and-reach | group | 1 | 6.50 | 0.02 | * | 0.32 | UT < T |
| menstrual phase | 2 | 1.93 | 0.16 | - | 0.12 | - | |
| group × menstrual phase | 2 | 1.28 | 0.29 | - | 0.08 | - | |
| - | - | - | - | - | - | - | - |
| Side-steps | group | 1 | 24.31 | 0.00 | ** | 0.63 | UT < T |
| menstrual phase | 2 | 0.84 | 0.44 | - | 0.06 | - | |
| group × menstrual phase | 2 | 0.03 | 0.97 | - | 0.00 | - | |
| - | - | - | - | - | - | - | - |
| 1000-m run | group | 1 | 94.90 | 0.00 | ** | 0.87 | UT > T |
| menstrual phase | 2 | 1.86 | 0.17 | - | 0.12 | - | |
| group × menstrual phase | 2 | 0.37 | 0.63 | - | 0.03 | - | |
| - | - | - | - | - | - | - | - |
| 50-m sprint | group | 1 | 32.78 | 0.00 | ** | 0.70 | UT > T |
| menstrual phase | 2 | 0.96 | 0.39 | - | 0.06 | - | |
| group × menstrual phase | 2 | 0.70 | 0.50 | - | 0.05 | - | |
| - | - | - | - | - | - | - | - |
| Standing long jump | group | 1 | 23.08 | 0.00 | ** | 0.62 | UT < T |
| menstrual phase | 2 | 0.81 | 0.46 | - | 0.05 | - | |
| group × menstrual phase | 2 | 1.21 | 0.31 | - | 0.08 | - |
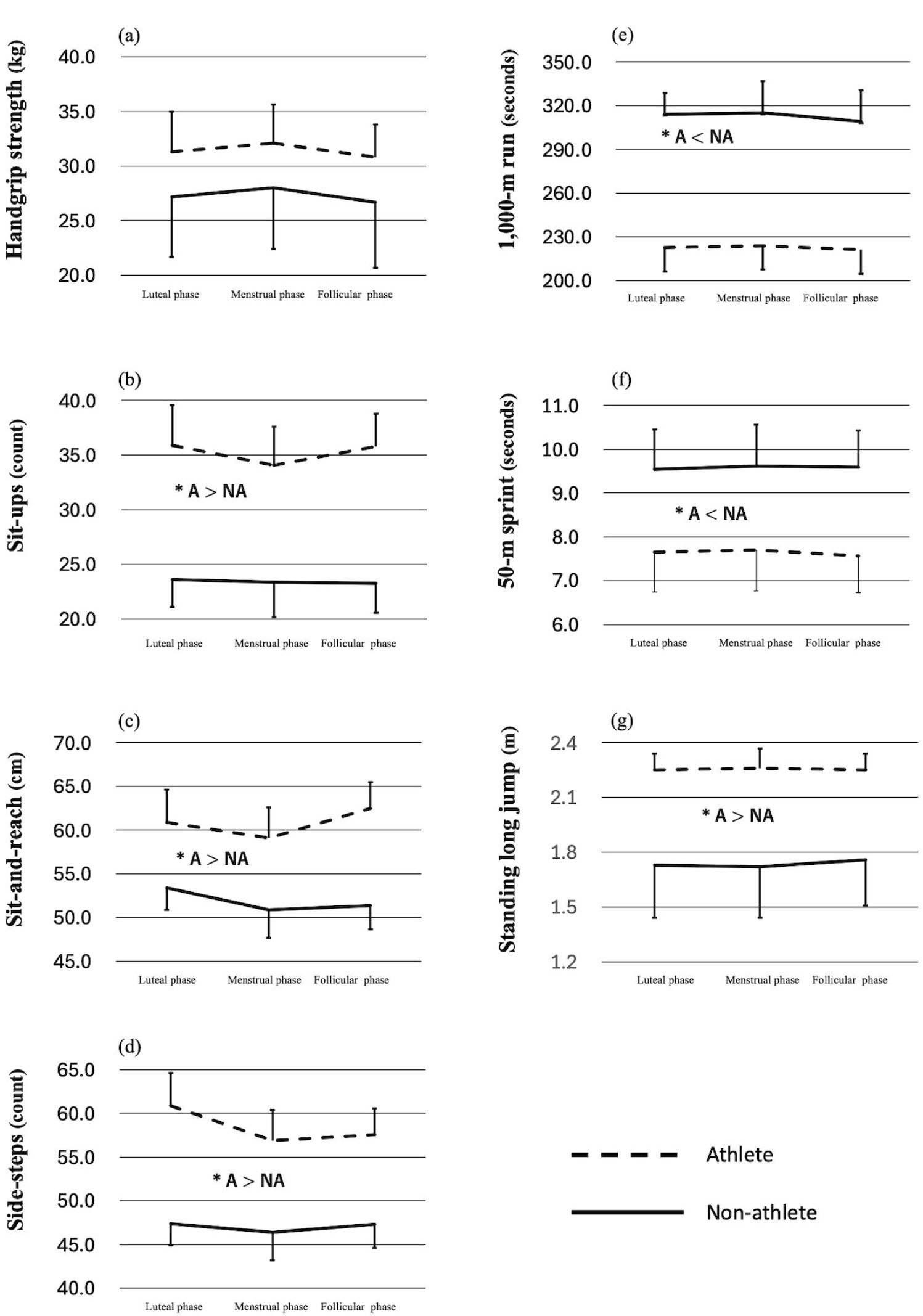
Interaction between menstrual phase and group (Athletes vs. Non-athletes) in exercise test results. (a) Handgrip strength, (b) Sit-ups, (c) Sit-and-reach, (d) Side-steps, (e) 1,000-m run, (f) 50-m sprint, (g) Standing long jump. A = Athletes; NA = Non-athletes. *p < 0.05, significant difference between groups.
| - | Menstrual Phase | Body Weight | Handgrip Strength | Sit-ups | Sit-and-reach | Side-steps | 1000-m Run | 50-m Sprint | Standing Long Jump |
|---|---|---|---|---|---|---|---|---|---|
| - | (kg) | (kg) | (count) | (cm) | (count) | (min:sec) | (s) | (cm) | |
| Non-athlete | Luteal | 57.4 | 25.3 | 27 | 65 | 44 | 5:10.74 | 9.97 | 154 |
| Menstrual | 57.4 | 29.0 | 27 | 65 | 42 | 4:58.72 | 10.69 | 155 | |
| - | Follicular | 57.3 | 27.2 | 27 | 62 | 46 | 4:50.46 | 9.91 | 178 |
| Athlete A | Luteal | 60.0 | 38.0 | 36 | 60 | 60 | 3:35.81 | 7.58 | 234 |
| - | Menstrual | 60.6 | 36.6 | 31 | 61 | 62 | 3:42.70 | 7.56 | 237 |
| - | Follicular | 60.7 | 32.8 | 35 | 66 | 59 | 3:38.28 | 7.55 | 233 |
| Athlete B | Luteal | 59.1 | 31.0 | 33 | 59 | 59 | 4:05.79 | 7.67 | 232 |
| - | Menstrual | 60.1 | 34.8 | 33 | 59 | 58 | 4:12.61 | 7.69 | 227 |
| - | Follicular | 59.6 | 32.3 | 34 | 60 | 60 | 4:12.74 | 7.51 | 229 |
Table 4 presents the fitness test results of participants with severe menstrual-related symptoms that affect their daily life. No consistent relationship was found between symptom severity and performance, either within or between individuals.
4. DISCUSSION
This study aimed to examine the influence of menstrual phase on fitness test performance and menstrual-related symptoms among female university students, comparing athletes and non-athletes. Although significant differences in physical symptoms were observed across menstrual phases in the athlete group and in both physical and psychological symptoms in the non-athlete group (as indicated by MDQ scores), no significant group-by-phase interaction or main effect of menstrual phase was found for any fitness test items. These results did not support our initial hypothesis.
Progesterone, which is secreted in larger quantities during the luteal phase, promotes fluid retention and may increase caloric intake through enhanced appetite mediated by insulin fluctuations [39, 40]. Previous studies have reported conflicting findings regarding menstrual cycle-related changes in body composition, with some observing significant fluctuations [29, 41] and others reporting no change [42, 43]. In the present study, no significant change in body weight was identified across the menstrual cycle in either group. Participants were instructed to maintain their usual diet, consistent with protocols in earlier studies. Considering that body weight regulation during the menstrual cycle may be influenced by hormonal shifts as well as interactions with other physiological factors, the lack of fluctuation seen in this study suggests that participants (regardless of training status) were not substantially affected by hormonal or related mechanisms.
Menstrual-related symptoms are known to affect physical, psychological, and social well-being. Consequently, a wide range of self-report instruments have been developed to assess such symptoms [4, 32, 34, 44]. These typically show that symptoms are most prominent in the late luteal phase, continuing into menstruation (with pain and other complaints) before subsiding. Several studies have demonstrated that exercise can alleviate symptoms [45] and that physically active women experience fewer or less severe symptoms compared to their sedentary counterparts [46-48]. Conversely, others have questioned the effectiveness of exercise in relieving menstrual discomfort. Studies such as those by Cicek [46] and Atan [49] reported that women without regular physical activity scored higher in “negative affect” and “lack of control.” Although the present study observed different symptom profiles between athletes and non-athletes, fewer than one-quarter of items showed significant changes in either group. Even the highest median score corresponded to a rating of “mild symptoms with no impact on daily life.” Overall, our sample can be characterized as a population with generally mild menstrual cycle-related symptoms.
Women who engage in regular training are well established as exhibiting superior physical capabilities, including muscular strength and cardiorespiratory function, compared to sedentary individuals. This finding was supported by the present study, which revealed significant main effects of group in all fitness tests except handgrip strength. Effect sizes between groups ranged from 1.09 to 5.9 across all menstrual phases, indicating robust performance differences. For handgrip strength, although the athlete group consistently achieved higher mean values and large effect sizes were observed (d = 0.86–0.88), statistical significance was not reached. This may reflect the limited statistical power of the present analysis (1 - β = 0.35), suggesting that the non-significant result could be attributable to a potential Type II error. Accordingly, handgrip strength should be considered as an important component to be examined more carefully in future studies with larger samples. These findings confirm that the athlete and non-athlete groups represented clearly distinct populations in terms of physical fitness. Considering body weight and MDQ scores together, both groups can be concluded to have comprised individuals minimally affected by the menstrual cycle.
The influence of the menstrual cycle on sports performance has been examined across various domains, as confirmed in multiple review articles [1, 3, 14-17, 50-52]. The present study assessed strength, muscular endurance, flexibility, agility, aerobic capacity, speed, and explosive power using a standardized fitness test protocol [35]. The use of such nationally standardized protocols —specifically, the “New Physical Fitness Test” administered annually by the Japan Sports Agency— represents a notable strength of the present study. This methodological approach enhances the reproducibility of our findings and represents a distinctive element compared to most previous investigations, as the administration of a nationally standardized fitness test is rare outside of Japan [53]. Importantly, even recent investigations have reported similar null findings; for example, Romero-Moraleda et al. observed no significant differences in neuromuscular performance and perceived exertion across menstrual cycle phases in elite female soccer players [54]. Velten et al. likewise found no phase-related differences in peak torque, fatigue indices, or subjective responses during isokinetic strength testing in resistance-trained women [55]. Although these reports primarily focus on athletic populations, they support the present findings. However, other studies have indicated that hormonal fluctuations, particularly in estrogen and progesterone, can affect neuromuscular activation [56-58], maximal oxygen consumption (VO2max) [59-61], and thermoregulation [1]. Janse de Jonge [15] therefore suggested that while certain phases may influence performance under specific conditions (e.g., long-distance events in hot environments), no consistent evidence has identified a menstrual phase that optimally enhances or impairs strength or aerobic capacity.
Several studies have reported menstrual cycle–related variations in muscle stiffness [62] and flexibility [63], while others have found no such changes [64, 65]. Increased knee laxity and patellar tendon extensibility have been observed in the luteal phase [19, 66]. However, tests like the sit-and-reach (comparable to the seated forward bend used in the present study) rely not only on hamstring extensibility, but also on thoracic and lumbar spine mobility [67], which may explain the lack of significant variation in our results. Similarly, although a previous study [26] reported reductions in agility during the premenstrual and early menstrual phases, the present study did not observe significant menstrual-phase effects. Interestingly, in the athlete group, a large effect size (d = 0.80) was observed between the luteal and menstrual phases in the side-step test, whereas non-athletes showed no such trend, and effect sizes remained small (d = 0.03–0.23). Given its popularity in Japan and routine use in schools, Sawai et al. [26] noted that this test may be sensitive to menstrual-related changes. Taken together with prior evidence that muscle stiffness in the joints involved in side-step movements is not influenced by menstrual phase [64], our findings do not allow any clear conclusions to be drawn regarding agility. In the present study, large effect sizes were observed in athletes only, whereas non-athletes consistently exhibited small effects. Although no significant interaction was detected, this group contrast may suggest the potential sensitivity of the side-step test for differentiating athletes from non-athletes in terms of menstrual-related changes. Such an interpretation can only be regarded as preliminary, however, given the lack of statistical significance and the limited sample size in the present study. This perspective may merit closer attention in future studies.
5. LIMITATIONS
This study has several limitations. First, from a methodological perspective, menstrual cycle phases were classified using BBT and symptom reporting. While practical, non-invasive, and low-cost, the method lacks precision and does not provide direct hormonal information. Combining BBT with hormonal assays has been recommended [2,3], and future studies should adopt such measures. In addition, although the modified MDQ has been applied in peer-reviewed research [29], its psychometric properties, including reliability and factor structure, remain untested and should be examined in future validation work.
Second, the sample size represents an important limitation. The effect sizes observed across menstrual phases were generally small, consistent with prior reviews [3, 12, 16, 17, 58, 66]. Detecting such subtle changes would require extensive samples, and is therefore unrealistic at a single site. Therefore, although the present study detected group-level differences, subtle fluctuations across menstrual phases may have gone undetected. In addition, 15 of the initial 31 participants withdrew during the study period, indicating that attrition bias should be considered when planning sample sizes in advance, as this may reflect injury incidence or within-subject fluctuations in physical condition.
Finally, individual cases in this study showed that performance variation can be clinically meaningful even within a small sample. Three participants reported severe symptoms, and in one athlete, a ~7-second difference in the 1,000-m run could have influenced competition ranking. Such findings, consistent with previous reports on PMS and dysmenorrhea [68, 69], highlight the need to focus not only on large-scale data, but also on women with pronounced symptoms. Future research should therefore involve multi-center collaborations, repeated measurements across multiple cycles within individuals, and targeted studies of women with more severe symptoms. These approaches may help define thresholds for the smallest clinically meaningful effects, contributing to more generalizable conclusions. Additionally, in the athletic context, coaches and athletes should recognize that the impact of menstrual cycle phases varies among individuals and should therefore tailor training regimens to the unique characteristics of each athlete.
CONCLUSION
This study investigated the effects of menstrual cycle phases on physical fitness test performance and menstrual-related symptoms among female university students, comparing athletes and non-athletes. Although both groups exhibited some changes in symptoms across menstrual phases, physical symptoms in athletes and both physical and psychological symptoms in non-athletes, no significant fluctuations in fitness performance were observed. These findings suggest that among women with mild premenstrual symptoms, the menstrual cycle may have a limited impact on physical fitness, regardless of training status. These outcomes might be relevant for physical fitness professionals and researchers, improving the understanding of the effects of menstrual cycle phases on physical fitness and psychological symptoms in women.
AUTHORS’ CONTRIBUTIONS
The authors confirm their contribution to the paper as follows: S.I.M.: Writing-Original draft preparation; D.A.M., H.P.N., A.R.A., R.F., K.M.: Writing-Reviewing and Editing. All authors reviewed the results and approved the final version of the manuscript.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study protocol was approved by the Research Ethics Committee of Tokyo Gakugei University (approval no. 636).
HUMAN AND ANIMAL RIGHTS
All procedures performed in studies involving human participants were in accordance with the ethical standards of institutional and/or research committee and with the 1975 Declaration of Helsinki, as revised in 2013.
CONSENT FOR PUBLICATION
All participants provided written informed consent after being informed of the experimental procedures and risks involved in the study.
AVAILABILITY OF DATA AND MATERIALS
All the data and supporting information are provided within the article.
CONFLICT OF INTEREST
Shin Ichiro Moriyama is the Associate Editorial Board member of the journal TOSSJ.
Henrique P. Neiva is a member of the Editorial Advisory Board of the journal TOSSJ.
Ana R. Alves is a member of the Editorial Advisory Board of the journal TOSSJ.
Ricardo Ferraz is a member of the Editorial Advisory Board of the journal TOSSJ.
ACKNOWLEDGEMENTS
We would like to thank Ms. Kyoko Tomizawa for her assistance in conducting the experiments.


