All published articles of this journal are available on ScienceDirect.
The Impact of Exercise with Blood Flow Restriction on Rectus Femoris and Vastus Intermedius Muscle Layer Thickness Following Anterior Cruciate Ligament Reconstruction: A Pilot Randomised Controlled Clinical Trial
Abstract
Introduction
This pilot randomised controlled trial assessed the feasibility and acceptability of incorporating blood flow restriction (BFR) training into the rehabilitation of anterior cruciate ligament reconstruction (ACLR) and evaluated its preliminary impact on muscle layer thickness (MLT) of the vastus intermedius (VI) and Rectus Femoris (RF) muscles.
Methods
Sixteen male participants who underwent ACLR with hamstring graft participated in a single-center, single-blinded pilot randomised controlled trial. Participants were randomly allocated into either a BFR group (28 ± 7 years) or a control group (23 ± 6 years). Both groups participated in a matching rehabilitation program (two sessions per week); the BFR group received BFR training, while the control group received a sham procedure. Ultrasonography was used to assess MLT of RF and VI pre-operatively, at 14 days, and 4 weeks post-operatively.
Results
Preliminary findings indicate that after 4 weeks, there were no significant differences in VI and RF MLT (p>0.05) following ACLR between the BFR and control groups.
Conclusion
Preliminary findings suggest that the addition of BFR training to rehabilitation following ACLR did not increase RF and VI MLT 4 weeks post-operatively. Future research should employ larger sample sizes and a longer intervention period to determine the effect of BFR training on VI and RF MLT following ACLR.
Clinical Trial Registration No.
The trial was registered with Research Registry with a unique number of researchregistry1061.
1. INTRODUCTION
Quadriceps Femoris (QF) muscle mass and strength are key indicators of stable knee function following Anterior Cruciate Ligament reconstruction (ACLR) [1-4]. However, due to the restrictive loading phase following knee surgery, QF atrophy is very common [5-7]. Recently, ischemic preconditioning (ischemia applied before exercise), ischemic postconditioning (ischemia applied after exercise), and ischemic exercise (blood flow restriction [BFR] during exercise) have been shown to attenuate atrophy and increase muscle size [8-14]. It is likely that ischemia causes an increase in growth hormone levels and collagen synthesis, thereby stimulating muscle metabolism and the production of new myocytes [15, 16]. However, whether BFR can enhance muscle size during Anterior Cruciate Ligament reconstruction rehabilitation (ACLRR) remains inconclusive.
BFR is likely to induce muscular adaptations linked to muscular hypertrophy or by reducing muscular atrophy [17]. Local hypoxia causes a mismatch between oxygen demand and supply, accelerating metabolic responses during ischemia. This leads to an anaerobic state, likely activating the secretion of growth hormones, resulting in increases in muscle fiber size (hypertrophy) [17-19]. Indeed, there are several reports of elevated secretions of growth hormones after BFR activation. Inagaki et al. [20], had 7 participants perform isometric knee contractions with electromyostimulation and repeated the exercise after 7 days with BFR. Blood samples were collected before and after the exercise. Results showed that noradrenaline increased similarly; however, elevated growth hormones were found during BFR sessions. Therefore, incorporating BFR in ACLRR might be beneficial for enhancing quadriceps muscle size.
Studies have shown diminished post-operative atrophy of the quadriceps following ACLR [12, 16], while others have reported no significant differences in quadriceps mass between the BFR training group and the control group following ACLR [14, 21]. The discrepancies between methods of exercise and BFR application make generalising reported results difficult. Moreover, no study has accounted for the individual differences that would cause discrepancies in results. Indeed, cuff width was a contributing factor in differences when applying BFR pressure [22, 23]. Clarkson and May [24] argue that BFR studies should justify the use of their BFR cuff pressure during application, whether for clinical or athletic purposes. Therefore, future research should incorporate a consistent methodological approach to assess whether BFR can enhance quadriceps muscle size, MLT, or reduce atrophy following ACLR. The purpose of this pilot study was to assess the feasibility of adding BFR training to rehabilitation of ACLR and evaluated the preliminary impact on MLT of VI and RF muscles.
2. MATERIALS AND METHODS
2.1. Study Design
This study is a pilot, prospective, single-center, single-blinded, randomised controlled clinical trial. Participants were blinded to the group they were assigned to. The first group was assigned the BFR application (BFR group), while the other group was assigned the same rehabilitation program in addition to a sham procedure (control group). Both groups participated in 8 sessions over 4 weeks of rehabilitation (2 sessions per week), following the same protocol with the same physical therapist at an outpatient physical therapy clinic in Alrazi National Hospital in Kuwait.
2.2. Randomisation
Participants were assigned a unique identification number using a mobile application that generated random numbers that resembled each participant in a 1:1 ratio to one of the two groups.
2.3. Inclusion and Exclusion Criteria
Inclusion criteria included patients aged 18 to 45 years with an ACL tear lasting more than 6 months, and a sufficient range of motion to perform exercise (active extension deficit < 5°, active flexion ≥120°). Also, the pain level during exercise was ≤2 on the Visual Analogue Scale (0–10 cm). Exclusion criteria included previous surgery on the affected knee, hypertension, intra-articular pathology that could alter rehabilitation or require open surgery or prolonged postoperative unloading, significant articular cartilage damage, additional neuromuscular impairments, spine or other lower limb injuries, and a history or presence of vascular disease or deep vein thrombosis.
2.4. Rehabilitation and Surgery Protocol
The surgical protocol involved an arthroscopic single-bundle ACLR performed with ipsilateral hamstring, and a thigh tourniquet was applied and inflated to a suprasystolic pressure of ~250mmHg. Femoral fixation was achieved using a cortical suspensory device (ACL-TightRope, Arthrex, Naples, FL, USA), and tibial fixation was secured with an absorbable interference screw (Biosure, Smith & Nephew, United Kingdom). The rehabilitation protocol involved an exercise regimen that included 2 sessions per week. All exercises prescribed were standardised as four sets of 30-15-15-15 reps, while rest was maintained at 30 to 60 seconds between sets. Rehabilitation consisted of two phases: phase 1 occurred from week 1 to week 3, and phase 2 commenced from week 3 onwards. During stage 1, participants engaged in quadriceps exercises, including isometric quadriceps contractions, leg extension over a knee-roll with no weight, and straight leg-raises in a supine position. These exercises progressed to a long sitting position, and then by adding weight. During stage 2, single-leg press, squats (0-30°) (Fig. 1), hamstring curls single leg, and calf raises were incorporated. Exercises that involved resistance were prescribed at 40% of 1 repetition maximum. Both groups were instructed to follow a home exercise program similar to the exercises prescribed in the clinic.

Participant performing mini squat exercise with blood flow restriction applied to the thigh as part of the rehabilitation following anterior cruciate ligament reconstruction.
2.5. BFR Application
For the BFR group, a cuff (Occlude Rehab+, Sweden) was wrapped around the proximal part of the operated thigh, and the cuff was inflated until reaching total arterial occlusion pressure (AOP) as mentioned elsewhere [25]. Cuff size (Small, Medium, Large, and Extra Large) used for the procedure varied according to the participant's thigh circumference. About 40-50% of AOP was used throughout the BFR program. To allow standardised pressure for all participants, a pocket doppler (EDAN SD3) with an 8 MHz probe was used to determine the AOP. Also, to maintain 40-50% of AOP, cuff pressure was verified using the sphygmomanometer after each exercise. Similarly, the same procedure was followed with the control group for detecting the AOP; however, a standardised pressure of 20 mmHg was administered as a sham among the participants.
2.6. Muscle Layer Thickness Assessment
The main outcome for comparing groups was MLT. Participants visited the radiology department 1-3 days before surgery (preoperative measurement), 14 days, and 4 weeks post-operatively. Transverse B-mode images were acquired using an ultrasound machine (Logiq E9; Medical Systems, Milwaukee, WI, USA) and a 10-MHz linear transducer. To maintain standardisation of MLT measurement, participants were present at the same time of the day during every ultrasound assessment visit. Participants were in a semi-supine position, hips in neutral position, and knees at full extension, while pillows were situated at the ankles to avoid any accessory movements during assessment. Using a tape measure, the area between the anterosuperior iliac spine and the lateral epicondyle was measured, and the midpoint was marked with a permanent marker to establish a standardised MLT assessment area.
A.A. conducted ultrasonic imaging of the RF and VI, with each muscle measured three times during the assessment session. At the same session, MLT was then obtained using an integrated ultrasound arrow (Fig. 2), to the nearest millimeter, and the median value was derived for the data analysis. The MLT of both RF and VI was determined by the distance between the two heads of the arrow.
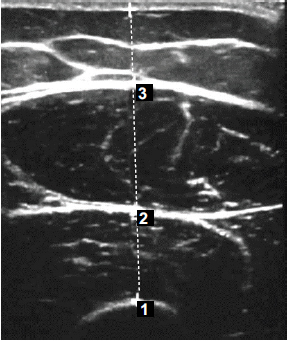
Ultrasound image of the anterior thigh 4 weeks following anterior cruciate ligament reconstruction.
2.7. Ethics
The study protocol was approved by the Medical and Health Ethics Committee of the Ministry of Health in Kuwait (2023/2221). All procedures were conducted in accordance with the Declaration of Helsinki.
2.8. Data and Statistical Analysis
Statistical analysis was performed using Statistical Package for the Social Sciences (IBM SPSS 28.0). Since the study was intended as a pilot, analyses were considered exploratory. Descriptive statistics were calculated for demographic variables and MLT. Normality of distribution was assessed using the Shapiro-Wilk test. A two-way measures ANOVA was conducted to assess whether there are significant main effects for condition (BFR x CONTROL) and time (pre-operatively, day 14, week 4). Data are displayed as mean ± SD, unless otherwise indicated. Differences were considered significant when p<0.05. An unpaired T-test was performed to analyse differences in MLT between the BFR and control groups after 4 weeks post-operation. CV and effect sizes were calculated as demonstrated elsewhere [26, 27]. Fig. (3) provides an illustration of the flow of participants through the pilot trial.
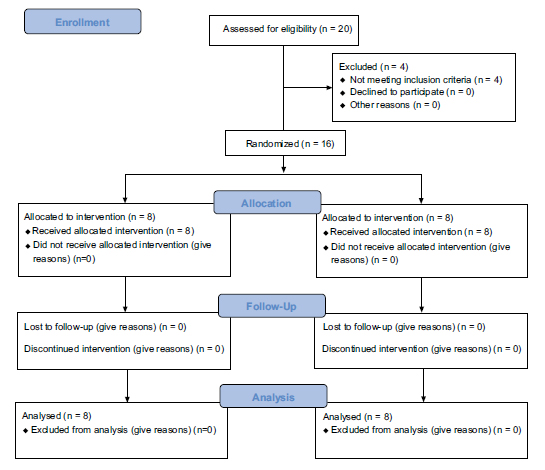
CONSORT flow diagram revealing participants flow through the study. The diagram shows enrollment, allocation, follow-up and analysis of participants.
3. RESULTS
3.1. Demographic Characteristics
Table 1 illustrates baseline demographic characteristics. In total, 16 participants (all males) participated in this study. All participants underwent ACLR with a hamstring graft performed by the same surgeons (N.N. and Y.S.). T-tests confirmed that there were no differences between the two groups in age, BMI, or MLT (p>0.05).
| Variable | BFR Group (Mean ± SD) |
Control Group (Mean ± SD) |
p-value |
|---|---|---|---|
| Age (years) | 28 ± 7 | 23 ± 6 | 0.1 |
| BMI (kg/m2) | 25 ± 5 | 29 ± 7 | 0.1 |
| RF MLT (cm) | 2.1 ± 0.6 | 2.4 ± 0.3 | 0.3 |
| VI MLT (cm) | 1.5 ± 0.5 | 2.2 ± 0.5 | 0.1 |
3.2. MLT Results
Table 2 displays the CV for all ultrasound measurements. Across all timepoints, CVs ranged from 1.2% to 6.2% for Rectus Femoris and from 1.2% to 7.1% for Vastus Intermedius, with mean CVs between 4.0% and 4.9% as displayed in Table 2. Table 3 reveals MLT results after conducting an exploratory two-way repeated ANOVA. The effect of time was significant across both groups, indicating loss of muscle thickness following surgery (RF, p=0.009; VI, p=0.036). However, no significant differences were observed in condition (RF, p=0.150; VI, p=0.437) or interaction across condition and time between both groups.
Fig. (4) demonstrates the results of MLT between both groups after 4 weeks post-operation. The exploratory T-test revealed no differences in MLT of RF after 4 weeks of BFR training between control (1.9 ± 0.2 cm) and BFR (1.8 ± 0.2 cm) groups (p= 0.30). Similarly, no differences were detected between MLT of VI between control (1.3 ± 0.2 cm) and BFR (0.9 ± 0.1 cm) groups (p= 0.25) (Fig. 5). Effect sizes were small for both VI and RF. For VI, it was -0.35 (95% CI: -1.33 to 0.65), and for RF, it was -0.26 (95% CI: -1.24 to 0.73).
| - | - | Preop | Day14 | Wk4 | Condition | Time | Interaction |
|---|---|---|---|---|---|---|---|
| RF MLT (cm) | CONTROL | 2.24 ± 0.4 | 2.01 ± 0.4 | 1.87 ± 0.5 | p=0.606 | p=0.002 | p=0.856 |
| BFR | 2.08 ± 0.5 | 1.89 ± 0.5 | 1.80 ± 0.5 | ||||
| VI MLT (cm) | CONTROL | 2.07 ± 0.5 | 1.43 ± 0.5 | 1.20 ± 0.5 | p=0.014 | p=0.002 | p=0.565 |
| BFR | 1.53 ± 0.5 | 0.97 ± 0.3 | 0.92 ± 0.2 |
| - | - | Mean CV (%) | Range (%) |
|---|---|---|---|
| RF | Preop | 4 | 1.2 – 5.9 |
| Week 4 | 4.6 | 1.3 – 6.2 | |
| VI | Preop | 4.6 | 1.1 – 6.8 |
| Week 4 | 4.7 | 1.2 – 7.1 |

Comparisons of MLT change (cm) of Rectus Femoris following anterior cruciate ligament reconstruction (ACLR). p-values represent two-way repeated ANOVA results for change in muscle layer thickness (MLT) after 4 weeks. Horizontal line represents mean.
4. DISCUSSION
The purpose of this pilot study was to assess the feasibility of incorporating BFR training into the rehabilitation of ACLR and evaluate the preliminary impact on MLT of VI and RF muscles. The main preliminary finding is that the trial is feasible, and exploratory analysis showed there were no differences in MLT of VI and QF measured by ultrasound 4 weeks following ACLR. Furthermore, effect sizes indicated minimal differences between groups in muscle thickness outcomes.
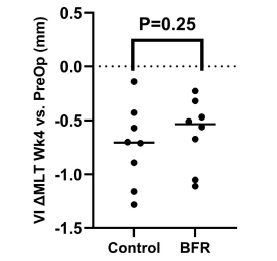
Comparisons of MLT change (cm) of Vastus Intermedius following anterior cruciate ligament reconstruction (ACLR). p-values represent two-way repeated ANOVA results for change in muscle layer thickness MLT after 4 weeks. Horizontal line represents mean.
There are several studies that have investigated the effect of BFR on QF muscle hypertrophy for ACLR patients. Takarada, Takazawa and Ishii [13], found that ischemic preconditioning diminished post-operative atrophy of knee extensors in ACLR patients. However, the short duration of the study made it difficult to show any clear insight into muscular adaptations following ACLR. Similarly, Ohta et al. [12], showed that training with BFR reduced knee extensor atrophy following ACLR. Cross-sectional areas of the knee extensor using MRI showed significant positive differences when compared to the control group at week 1 and week 16 postoperatively. However, Iversen et al. [21], showed that BFR did not increase knee extensor CSA 16 days after ACLR, as revealed by MRI investigations. Moreover, Zargi et al. [14], found no differences in QF mass using MRI between patients who underwent BFR with low-load exercise and those with conventional rehabilitation following ACLRR at 12 weeks post-ACLR. Another reason can be attributed to the exercise parameters used in studies investigating BFR in ACL patients. Iversen et al. [21], instructed patients to perform isometric quadriceps contractions, leg extension over a knee-roll, and straight leg raises adding up to 200 repetitions per exercise per day. While Ohta et al. [12], instructed patients to perform straight leg raises, hip abduction exercises, hip adduction exercises by holding a ball between both knees, and quadriceps isometric contractions. Further, 20 repetitions were recommended, with each contraction maintained for 5 seconds. These discrepancies in results can be attributed to differences in methodologies that were used in these studies. Therefore, we employed a standardised methodology to have more definite conclusions on the effects of BFR on QF following ACLR.
In the present study, preliminary results showed no differences in the MLT of VI and RF after 4 weeks post-ACLR. These results are consistent with Zargi, et al. [14], Iversen et al. [21], who found no differences in QF size between BFR and conventional rehabilitation when using MRI to assess cross-sectional areas of the QF. However, both studies used different BFR protocols. Zargi et al. [14], used BFR during the last 10 days before surgery, while Iversen et al. [21], used 5 cycles of inflation and deflation twice a day for 2 weeks after surgery. In our study, we employed an individualised AOP application using duplex ultrasound to minimise variation that might affect occlusion pressure, such as cuff size or blood pressure [17, 24, 28]. This approach is considered a strength of the study because it accounts for individual differences when determining BFR pressure. Indeed, inconsistencies in BFR protocols across studies highlight the importance of a standardized method to maximise the benefits of BFR in rehabilitation. Moreover, using individualised pressure overcomes the limitations associated with applying fixed pressure.
Therefore it is important to justifying cuff pressure as underlined by Clarkson et al. [24], who have found only 7 out of 52 studies that justified their cuff pressure chosen in their studies. They suggest researchers to justify the use of pressure and called for an individualised approach for BFR application. Failing to justify pressure used within the study makes comparisons between studies difficult, and attempts to standardise parameters for better patient outcomes remain limited.
Previous studies have shown promising results regarding the effects of combining BFR with conventional rehabilitation for musculoskeletal patients’ muscle hypertrophy [8-10]. Shinohara et al. [29], reported a 26% increase in maximal voluntary contraction in subjects after 4 weeks of training with a 250 mmHg inflated cuff around their thighs compared to subjects who trained similarly without BFR. Conversely, BFR showed mixed results for muscle strength and size in patients following ACLR [11-14, 21]. Some studies [12, 13], have reported reductions in QF atrophy, while others [14, 21], have found no differences in QF size between the BFR and conventional rehabilitation groups. This may be attributed to the heterogeneity in methodological approaches that have been used and the limited studies that have investigated the combination of BFR with conventional rehabilitation following ACLR.
On the other hand, studies have shown reductions in atrophy following ACLR in QF with a standard fixed pressure. Interestingly, an earlier study by Takarada et al. (2000) [13], found reductions in QF trophy (9% BFR group vs 21% control group) 16 days following ACLR using an intermittent protocol of 5 cycles of inflation (180mmHg) and deflation twice a day post ACLR. Ohta et al. (2003) also found reductions in QF atrophy (9% BFR vs 21% control) using 20% of 1 RM and a standardised 180mmHg cuff inflation while training for 16 weeks. These differences in results compared to our study can be attributed to methodological discrepancies. We have employed an individualised BFR pressure for each participant, while a fixed pressure was used by Ohta and Takarada et al. (2000). Hence, the importance of careful consideration of parameters, such as pressure application, when using it for rehabilitation protocols is evident [12, 13].
In our study, we have attempted to standardise the BFR protocol by using an individualised, consistent cuff pressure and duration of restriction. This study has attempted to test whether BFR might enhance VI and RF MLT following ACLR. At these levels of BFR application, preliminary results showed no differences in MLT between the control group and the BFR. Several explanations might contribute to these preliminary findings. BFR levels used in this study might not be a sufficient trigger for positive effects on muscle MLT. However, a review by Das and Paton [25] suggested that the ‘optimal zone’ for eliciting benefits of BFR was around %LOP of 40-150% and no lesser than 30% of 1RM, which is consistent with the parameters that have been used in this study. Another reason that might explain these results is that the window of muscle adaptations was not sufficient to occur. The MLT recording of 4 weeks might not be sufficient to elicit such responses. Indeed, Takarada et al. (2000) and Ohta et al. (2003) used BFR for more than 14 weeks, where they found evidence of QF atrophy reductions. This may suggest that extended periods of BFR application might be needed to enhance muscle hypertrophy or MLT. Nevertheless, previous reports have shown increases in muscle size after at least 14 days of BFR application [12, 13].
4.1. Study Limitation
There are several limitations associated with this study. Firstly, the pilot nature and small sample size of this study make it difficult to draw definitive conclusions about the impact of BFR training on VI and RF atrophy following ACLR. The other limitation is that these results pertain to two muscles (VI and RF) that form the QF, so the BFR effect on the other muscles of the quadriceps femoris (Vastus Medialis and Vastus Lateralis) remains unknown. However, the rationale behind VI and RF selection is related to the accessibility and position of VI and RF, making clearer visualisation during ultrasound measurement [30, 31]. Furthermore, VI and RF are commonly affected following ACLR, in addition to their established reliability and utility during ultrasound measurements after ACLR [32, 33].
Furthermore, the values of CV displayed in this study indicate good reliability of our ultrasound method, consistent with previous reports [34, 35]. This is a limitation associated with using ultrasound imaging versus Magnetic Resonance Imaging (MRI), which allows the visualisation of the cross-sectional area and in-depth measurements and is considered far superior. However, ultrasound measurements are considered reliable and valid when compared to MRI imaging for muscle size measurements [36, 37]. Another limitation is that the study was conducted in a single centre, and this may reduce the generalisability of the findings to different settings that would use BFR for rehabilitation purposes. Another limitation of this study was concerned with gender. This study included only males as participants. Therefore, it is difficult to generalise these results to the other gender. Another limitation related to this study is the short intervention period of 4 weeks, which may not be sufficient to elicit hypertrophy. However, studies have shown changes using BFR in similar time frames [13, 25]. Therefore, future studies should account for the duration of the intervention and include assessments of Vastus Lateralis and Vastus Medius. Additionally, including females would be advisable to obtain more generalisable findings concerning BFR application for rehabilitation purposes after ACLR.
CONCLUSION
In conclusion, preliminary findings suggest that the combination of BFR with conventional rehabilitation at the applied intensity of 40-50% of AOP for 4 weeks does not significantly increase MLT of RF and VI compared to conventional rehabilitation alone during early stages of rehabilitation. However, this pilot study demonstrated the feasibility of the methods and the success of the personalised approach to BFR application. Furthermore, future research should employ larger sample sizes and a longer intervention period to determine the effect of BFR training on VI and RF atrophy following ACLR.
AUTHORS’ CONTRIBUTIONS
The authors confirm their contributions to the paper as follows: J.A.: Study conception and design; S.A.: Conceptualization; A.A. and D.A.: Data collection; A.L. and N.A.: Methodology; H.A.: Visualization; A.S.: Analysis and interpretation of results; T.A. and Y.A.: Writing - Original draft preparation. All authors reviewed the results and approved the final version of the manuscript.
LIST OF ABBREVIATIONS
| BFR | = Blood Flow Restriction |
| ACLR | = Anterior Cruciate Ligament Reconstruction |
| MLT | = Muscle Layer Thickness |
| VI | = Vastus Intermedius |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study was approved by the Research Ethics Review Committee of the Kuwait Ministry of Health, Kuwait (2023/2221).
HUMAN AND ANIMAL RIGHTS
All human research procedures followed were in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national), and with the Helsinki Declaration of 1975, as revised in 2013.
CONSENT FOR PUBLICATION
Written informed consent was obtained from the patient for publication of this case report and accompanying images.
AVAILABILITY OF DATA AND MATERIALS
The data and supportive information are available within the article.
ACKNOWLEDGEMENTS
Declared none.


