All published articles of this journal are available on ScienceDirect.
CaRiSMA 1.0: Cardiac Risk Self-Monitoring Assessment
Abstract
Background:
Sport-related sudden cardiac death (SRSCD) can only be fought through prevention.
Objective:
The aim of this study is to propose an innovative software application, CaRiSMA 1.0 (Cardiac Risk Self-Monitoring Assessment), as a potential tool to help contrasting SRSCD and educating to a correct training.
Methods:
CaRiSMA 1.0 analyzes the electrocardiographic and heart-rate (HR) signals acquired during a training session through wearable sensors and provides intuitive graphical outputs consisting of two traffic lights, one related to cardiac health, based on resting QTc (a parameter quantifying the duration of ventricular contraction and subsequent relaxation), and one related to training, based on exercise HR. Safe and worthwhile training sessions have green traffic lights. A red QTc traffic light indicates the need of a medical consultation, whereas a red HR traffic light indicate the need of a reduction of training intensity. By way of example, CaRiSMA 1.0 was applied to sample data acquired in 10 volunteers (age= 27±11 years; males/females 3/7).
Results:
Two acquisitions (20.0%) were rejected because too noisy, indicating that wearable sensors may record poor quality signals. The QTc traffic light was red in 1 case, indicating that people practicing sport may not be aware of being at risk. The HR traffic light was red in 0 cases.
Conclusion:
CaRiSMA 1.0 is a software application that, for the first time in the sport context, uses QTc, the most important index of cardiac risk in clinics. Thus, it has the potential for giving a contribution in the fight against SRSCD.
1. INTRODUCTION
Sport-related sudden cardiac death (SRSCD) is a “death occurring during sport or within one hour of cessation of sports activity” [1]. Although representing the leading medical cause of death in athletes, SRSCD exact incidence remains unclear but likely higher than traditional estimates [2]. SRSCD occurs in the presence of underlying cardiovascular diseases [3, 4]. Thus, specific screening programs for identification of cardiovascular abnormalities predisposing to SRSCD should be recommended to all athletes [5-7], while usually conceived only for competitive athletes. Instead, amateur athletes and people occasionally practicing sport are typically left to optional self-monitoring evaluations of their health. Popular wearable physical-activity monitoring sensors are typically used to optimize exercise dose for increasing fitness level. Still, the same biomedical data used for training optimization could easily provide useful information for non-invasive self-evaluation of SRSCD risk [8]. So far, only cardiovascular parameters related to heart rate (HR), such as exercise HR, have been used to improve performance and monitor health [8]. Electrocardiographic (ECG) QT-interval is the most popular ECG marker of cardiac risk in clinics and the standard indicator of cardiac safety in clinical trials [9-12], especially in its HR-corrected form (QTc) [13]. As far as we know, it has never been used in sports applications finalized to large-scale prevention, even though some modern wearable sensors record the ECG signal and retrospective observational studies on professional athletes have indicated a prevalence of prolonged QTc [14-19].
The aim of this methodological work is to propose CaRiSMA 1.0 (Cardiac Risk Self-Monitoring Assessment) as a tool with the dual scope of helping contrasting SRSCD and educating to a correct training. CaRiSMA 1.0 is an innovative user-friendly software application to be associated to wearable sensors that allows cardiac-health self-evaluation by analyzing resting QTc and exercise HR.
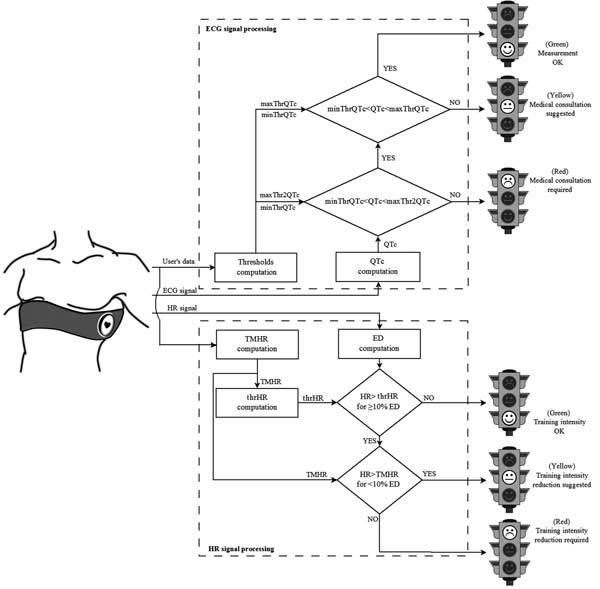
2. METHODS
2.1. CaRiSMA 1.0 Application Overview
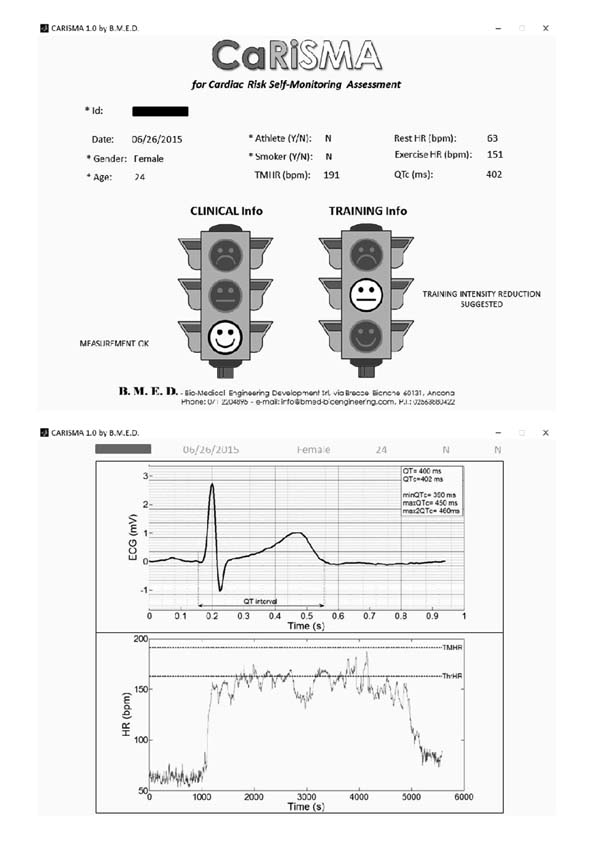
CaRiSMA 1.0 is an application for self-monitoring of cardiac health. It is mainly (but not necessarily) thought for amateur athletes and people occasionally practicing sport (from now on termed “users”) of any age and education, better if performing aerobic exercise. While training, the user wears a sensor able to record the ECG signal and, eventually, the HR signal. After training, such signals are downloaded on a personal computer to be analyzed Fig. (1).
Its inputs include the user’s data (age, gender, smoking habits, practiced sport, and athletic level) and signals (the mandatory ECG signal and the optional HR signal). For reliability, only good quality ECG signals are further processed [20]. If the HR signal was not provided, CaRiSMA 1.0 automatically derives it from the ECG signal.
CaRiSMA 1.0 main output (see sample runs below) is graphical and consists of two traffic lights, one related to resting QTc and the other to exercise HR. Resting-QTc traffic light provides information about the possible presence of cardiac abnormalities which might require a medical doctor consultation: green indicates that the QT measurements are ok, yellow that a medical consultation is suggested, and red that a medical consultation is required. Instead, exercise-HR traffic light suggests user-driven training actions: green indicates that the training intensity is ok, yellow that a reduction of training intensity is suggested, and red that a reduction of training intensity is required. In addition, CaRiSMA 1.0 provides a graphical output showing the representative ECG beat from which the QT interval was computed, and the HR signal. A numerical output containing the parameters used in the processing is also provided.
2.2. CaRiSMA Methodology
2.2.1. Measurement Protocol
To use CaRiSMA 1.0, cardiac data should be acquired with any adequate wearable sensor according to the following protocol:
- Step 1: Position and start the wearable sensor.
- Step 2: Remain in resting conditions (sitting or lying); resting duration (RD) should last at least 5 minutes.
- Step 3: Exercise; exercise duration (ED) is free.
- Step 4: Optionally (suggested), acquire cardiac data while recovering; recovery duration should last at least 5 minutes.
- Step 5: Take off the sensor and download data on a personal computer.
- Step 6: Run CaRiSMA 1.0.
2.2.2. QTc Traffic Light
CaRiSMA 1.0 implements the Zhang et al.’s method for a reliable automatic QT-interval measurement [21]. A detailed description of this method is beyond the scope of this work and can be found elsewhere [21]. The QT interval is defined as the time interval between ECG Q-wave beginning and T-wave end, and quantifies the duration of ventricular contraction and subsequent relaxation. Abnormal QT intervals may be due to both acquired (drugs, pathological conditions, etc.) and genetic causes, and represent abnormalities in the electrical recharging system of the heart, whose structure remains normal. Both lengthened and shortened QT intervals are associated to an increased risk of developing malignant ventricular arrhythmias or fatal ventricular fibrillations. The QT interval is HR dependent for which is typically corrected (QTc, ms) using the Bazett's formula [13]:
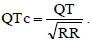 |
(1) |
In clinics, cardiac risk is accomplished by comparing measured QTc against the values reported in the QTc scales proposed by the Food & Drug Administration (FDA) for non-athletes [12, 22], and by the Seattle Criteria for athletes [23] (Table 1).
| Proposed by | QTc (ms) | Male | Female |
|---|---|---|---|
| FDA (for non-athletes) |
Long QT | >450 | >460 |
| Possible Long QT | 431-450 | 451-460 | |
| Normal | 390-430 | 390-450 | |
| Short QT | <390 | <390 | |
| Seattle Criteria (for athletes) |
Long QT | >499 | >499 |
| Possible Long QT | 470-499 | 480-499 | |
| Normal | 321-469 | 321-479 | |
| Short QT | <321 | <321 |
CaRiSMA 1.0 uses such scales to compute three user-specific thresholds: minThrQTc, which discriminates short from normal QT intervals; maxThrQTc, which discriminates normal from possibly prolonged QT interval; and maxThr2QTc, which discriminates possible prolonged from prolonged QT intervals. The numerical values of such thresholds are determined as follows:
 |
(2) |
 |
(3) |
 |
(4) |
Resting QTc, obtained by correcting the QT interval of the median beat computed using all beats in the initial phase of resting (Eq. 1), is then compared against such thresholds Fig. (1). If QTc≤minThrQTc (short QT) or QTc≥maxThr2QTc (long QT) a medical consultation is required (red traffic light). Differently, if QTc≥maxThrQTc (possible long QT) a medical consultation is suggested (yellow traffic light); otherwise the QT measurement is ok (green traffic light).
2.2.3. HR Signal Traffic Light
Theoretic maximum HR (TMHR; bpm) is one of the most commonly used features for prescribing safe exercise intensity. CaRiSMA 1.0 computes TMHR according to the athlete smoking habits [24]. For non-smoking individuals with no overt cardiovascular disease and taking no medications, TMHR can be computed as [25]:
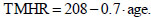 |
(5) |
Instead, for smoking healthy subjects, TMHR is computed as [24, 26]:
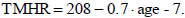 |
(6) |
Eventually, in the presence of cardiovascular diseases and/or when using medications, CaRiSMA 1.0 computes no TMHR, since its value may depend on the type of disease or medication; users should use CaRiSMA 1.0 under medical supervision and the physician should determine his/her optimal TMHR to be set in the application (by providing it with the user’s data). Recommended target HR zone is 50 to 85% of TMHR. Exceeding 85% of TMHR can be dangerous as exercising at an extreme intensity increases the risk of cardiac events [27], besides increasing the risk of over-training, injury and illness. Thus, CaRiSMA 1.0 defines a user-specific HR-related threshold (thrHR) as 85% of TMHR, which is the highest HR of the recommended target zone:
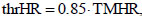 |
(7) |
and compares HR during exercise against it Fig. (1). If HR never exceeds thrHR, or exceeds it for less than 10% of ED, the training intensity is ok (green traffic light); otherwise, if HR never exceeds TMHR, or exceeds it for less than 10% of ED, a reduction of the training intensity is suggested (yellow traffic light); eventually, if HR exceeds TMHR for at least 10% of ED a reduction of the training intensity is required (red traffic light).
2.3. CaRiSMA 1.0 Input/Output Files Specifications
After having manually inserted the athlete’s id (together with all other user’s data), CaRiSMA 1.0 automatically looks for, loads and processes id_ECG.csv and id_HR.csv files. Other accepted files extensions are *.txt, *.mat and *.xls. Then, CaRiSMA 1.0 provides: id_CaRiSMAreport.bpm, a graphical output mainly showing the traffic lights with the associated suggestions (Fig. (2) upper panel); id_CaRiSMAplots.bpm, a graphical output mainly showing the representative ECG beat and the HR signal (Fig. (2) lower panel); and id_CaRiSMAoutparameters.txt, a numerical file containing the values of minThrQTc, maxThrQTc, maxThr2QTc, resting QT, resting QTc, resting HR (mean HR over the resting), exercise HR (mean HR over exercise), maximum HR, TMHR and thrHR.
2.4. CaRiSMA 1.0 Sample Study
At the present time, a statistical evaluation on the utility of CaRiSMA 1.0 in preventing SRSCD is premature, since it would require a wide-spread use of the application or the availability of a large collection of data acquired according to its specification (such database is actually under construction in our lab). The data from the subjects used here are not intended for statistical evaluations, but for sample representations of CaRiSMA 1.0 runs.
Data were acquired in 10 volunteers (age= 27±11 years; 3 males and 7 females; 1 smoker; 5 runners, 4 tennis players and 1 aerial silks dancer), of which 5 were athletes (i.e. practicing sport regularly and possibly doing competitions) while the remaining 5 practiced physical activity only occasionally (Table 2). All participants signed an informed consent. The study was approved by the institutional expert committee. Acquisitions were performed using the BioHarness 3 by Zephyr (www.zephyranywhere.com), that provides both ECG (sampling rate: 250 Hz) and HR signals (sampling rate: 1 Hz). Volunteer n. 6 was monitored three times to show CaRiSMA 1.0 short- and long-term repeatability; the second and third acquisitions were close in time (3 days) and occurred after more than four months from the first one.
| Sbj | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
|---|---|---|---|---|---|---|---|---|---|
| User’s data |
Age (years) |
24 | 26 | 22 | 24 | 23 | 57 | 25 | 22 |
| Gender (M/F) |
F | M | F | F | F | M | F | F | |
| Smoker (Y/N) |
N | N | N | N | N | N | Y | N | |
| Sport | Aerial Silks | Running | Running | Running | Running | Tennis | Tennis | Tennis | |
| Athlete (Y/N) |
Y | N | N | N | N | Y | Y | Y | |
| Graphical output |
QTc traffic light |
 |
 |
 |
 |
 |
 |
 |
 |
| HR traffic light |
 |
 |
 |
 |
 |
 |
 |
 |
|
| ECG beat (µV) |
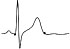 |
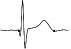 |
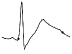 |
 |
 |
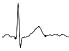 |
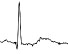 |
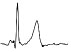 |
|
| HR trend (bpm) |
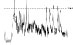 |
 |
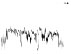 |
 |
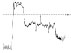 |
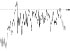 |
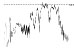 |
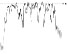 |
|
| Numerical output |
QTc (ms) |
403 | 412 | 577 | 402 | 417 | 405 | 469 | 387 |
| Resting HR (bpm) |
87 | 98 | 85 | 63 | 72 | 81 | 104 | 77 | |
| Exercise HR (bpm) |
129 | 135 | 105 | 151 | 164 | 143 | 146 | 174 | |
| Max HR (bpm) |
226 | 184 | 128 | 187 | 238 | 190 | 190 | 198 | |
| TMHR (bpm) |
191 | 190 | 194 | 191 | 192 | 168 | 184 | 193 |
3. RESULTS: CaRiSMA 1.0 SAMPLE STUDY
In this section are reported results related to CaRiSMA 1.0 sample study. Such results represent examples of CaRiSMA 1.0 application and are not intended for deriving general statistical conclusions on specific populations of athletes. Still, they may suggest interesting issues relative to amateur athletes and people occasionally practicing sports.
Two acquisitions out of 10 (20%) were rejected because too much noise affected the ECG signal jeopardizing reliability (low signal to noise ratio), indicating that the quality signal recorded by wearable sensors are not always as good as expected [28]. Results relative to the remaining 8 volunteers are reported in Table 2.
The QTc traffic light was red in 1 case (who actually showed an abnormal ECG beat), yellow in 0 cases and green in 7 cases. Instead, the HR traffic light was red in 0 cases, yellow in 6 cases and green in 2 cases (with default TMHR settings). In only one volunteer (n. 2) both traffic lights were green. The complete CaRiSMA 1.0 graphical report of volunteer n. 4 is shown in Fig. (2), while miniatures of CaRiSMA reports of all subjects are reported in Table 2 to explain reported traffic lights color.
Results relative to the short- and long-term repeatability in volunteer n. 6 are reported in Table 3: QTc values are normal and comparable in length in all acquisitions, so that 3 green QT traffic lights were obtained. Instead, training intensity (HR levels) has grown with time, providing a green HR traffic light in the first acquisition and a yellow one in the following two.
| Acquisition date | 10/17/2015 | 02/29/2016 | 03/02/2016 | |
|---|---|---|---|---|
| Graphical output |
QTc traffic light |
 |
 |
 |
| HR traffic light |
 |
 |
 |
|
| ECG beat (µV) |
 |
 |
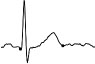 |
|
| Graphical output |
HR trend (bpm) |
 |
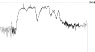 |
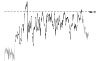 |
| Numerical output |
QTc (ms) |
417 | 420 | 405 |
| Resting HR (bpm) |
82 | 91 | 81 | |
| Exercise HR (bpm) |
122 | 129 | 143 | |
| Max HR (bpm) |
156 | 164 | 190 | |
| TMHR (bpm) |
168 | 168 | 168 |
4. DISCUSSION
This work proposes CaRiSMA 1.0 as a possible tool to contrast SRSCD and educate to a correct training. Specifically, QTc analysis is performed for contrasting SRSCD, whereas HR analysis is performed for training education. CaRiSMA has been developed for subjects practicing sport at all levels: from those who approach it occasionally, to amateur athletes to competitive and professional athletes. It is a software application that needs to be used in association with wearable sensors providing at least the ECG signal. Commercial applications based on HR for training optimization are quite common. However, to our knowledge, CaRiSMA 1.0 introduces for the first time the measure of the QT-interval (the major index of cardiac risk in clinics [9-12]) in a sport application finalized to large-scale prevention, which is the only weapon to fight SRSCD [29]. This feature makes CaRiSMA 1.0 very innovative. The QT inclusion in the application is supposed to be very promising, since previous studies on professional athletes indicated a prevalence of prolonged QTc [14-19]. Thus, the innovation provided by CaRiSMA 1.0 is not in the methodological content, that is well known in clinics, but rather in its application to the sport context.
CaRiSMA 1.0 was especially thought for occasional to amateur subjects practicing sports (since they usually do not undergo medical evaluations) and aims to educate them to a correct training planning and to monitor their need of a physician consultation. Education is indeed considered the key to fight SRSCD [6]. CaRiSMA 1.0 can also be used by competitive athletes, for whom QTc and HR safety regions can be manually adjusted by sport doctors or trainers since it is well known that there is a physiological cardiac adaptation to regular intense exercise [30].
4.1. CaRiSMA 1.0 Methodology
Although based on solid scientific knowledge, CaRiSMA 1.0 is not a medical tool but, rather, a tool to facilitate identification of athletes of any level at increased risk that may require medical consultation, beside training adjustments. These messages are provided by means of traffic lights that provide a simple and intuitive graphical output to educate people practicing sport to correct cardiac-health monitoring and training planning. Specifically, the output resting-QTc traffic light provides information about the need of a cardiologist consultation, whereas the exercise-HR traffic light provides suggestions about how to modify training. Safe and worthwhile training sessions have green traffic lights.
The QT traffic light color is based on the measure of the QT interval automatically performed by the Zhang et al.’s method [21], which is a robust technique for reliable QT interval measurement, as demonstrated by experimentations with the PhysioNet QT database (http://www.physionet.org/physiobank/database/qtdb), which also showed that it outperformed other published algorithms evaluated on the same database [21]. The algorithm was also used in a meta-analysis study on mortality associated to the QT interval [10], which reported consistent associations between prolonged QT interval and increased risk of sudden cardiac death, and recognized the QT-interval as a determinant of mortality in the general population. Our implementation of the Zhang et al.’s method was previously tested in a clinical study [31].
The HR traffic light color is based on the value of exercise HR, that is then compared to a threshold (thrHR) obtained using TMHR (Eq. 7). Several formulas have been proposed in the literature to compute TMHR [25, 32-36], the most popular of which remains 220-age [33]. This formula, however, underestimates TMHR in young adults and does not consider smoking habits, cardiovascular diseases and use of medications as independent factors [25, 35, 37]. Thus, for non-smoking individuals with no overt cardiovascular disease and taking no medications, the formula reported in Eq. 5 was considered. Such TMHR formula was first obtained through a meta-analysis including 351 studies, 492 subject groups and 18,712 subjects; then, it was cross-validated in a controlled, laboratory-based study involving 514 healthy subjects (18-81 years old) [25]. Results indicated that Eq. 5 can be used to estimate TMHR independently of gender and physical activity status. Two independent studies on 298 young adults (20-29 years old) and 1960 middle aged subjects (40-59 years old) have shown that smokers, during exercise, reach their maximum HR with more difficulty than non-smokers [24, 26]. Specifically, on average, smokers had a lower TMHR by 7 bpm. Consequently, for smoking healthy subjects, TMHR was estimated as in Eq. 6. TMHR is widely used in the physical fitness industry to gauge exercise intensity [38]; however, none of the formulae used for its estimation, included the ones implemented in CaRiSMA 1.0, are particularly accurate, especially because not taking into account the significant HR variability among individuals [18]. Consequently, in some cases, as for professional athletes or when following specific training plans [39], exceeding TMHR is not necessarily a problem. In case of self-monitoring amateur or occasional athletes, however, the main goal is not exact determination of TMHR but, rather, identification of safe ranges of HR in which the user should stay while exercising; under these circumstances a TMHR estimate may still be useful.
Besides providing QTc and HR traffic lights, CaRiSMA 1.0 also provides another graphical output, the representative ECG from which beat the QT interval is computed and the HR signal (Fig. (2) lower panel). This additional output allows more expert subjects (such as medical doctors and/or trainers) to critically check and interpret CaRiSMA 1.0 results. Eventually, CaRiSMA 1.0 provides a numerical file (id_CaRiSMAoutparameters.txt) containing the values of several parameters (physiological and not) in order to allow future retrospective studies on the single athlete or on teams.
4.2. CaRiSMA 1.0 Sample Study
In our sample study, 20.0% of the data had to be rejected because too noise. This percentage is quite important but cannot be addresses to CaRiSMA but to the wearable sensors that not always acquire data with the claimed precision. In addition, it underlies the fact that physiological signal recordings through some commercial wearable sensors may not be of sufficiently good quality for clinical evaluations [28]. Several studies showed that many non-invasive wearable sensors have questionable validity and reliability when used in various sport settings and populations [8, 40, 41]. To reduce the number of unusable acquisitions due to noise (20.0% in our sample runs), CaRiSMA 1.0 should always be combined with good quality sensors, and athletes should put a lot of care in correctly wearing them.
Our sample study also reported one case out of 8 (12.5%) of red QTc traffic light (result visually confirmed by ECG analysis; Table 2) suggesting that the need of a medical consultation may be much more frequent than expected. Eventually, 75.0% of volunteers had a yellow HR traffic light, suggesting that amateur or occasional people practicing sport may not realize that they reached too high HR during training. Consequently, they may not be aware of being at risk during a training session, until they suddenly feel sick; in the worst cases, the outcome is SRSCD.
4.3. CaRiSMA 1.0 Software
CaRiSMA 1.0 outputs are provided after a training session by running the application on a computer. CaRiSMA 1.0 was developed using MATLAB® 7.6.0 (R2008a) and compiled to a deployable standalone application with the MATLAB® Compiler 4.8. The MATLAB® Compiler Runtime 7.8 is required for running CaRiSMA 1.0. CarRiSMA 1.0 can be obtained by directly contacting the responsible of the research group (Laura Burattini, l.burattini@univpm.it). Collaborations finalized to CaRiSMA 1.0 utility evaluation and clinical studies are more than welcome.
Development of a CaRiSMA 1.0 app providing alarms in real time is in progress. Future versions of CaRiSMA using other innovative ECG risk indexes such as T-wave alternans and f99 [20, 42, 43], besides QTc, are also under evaluation.
CONCLUSION
CaRiSMA 1.0 is a software application for cardiac-health self-evaluation besides training education. For the first time in the sport context, it uses QTc, the most important index of cardiac risk in clinics, and thus has the potential for giving a significant contribution in the fight against SRSCD.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study involved healthy 10 volunteers practicing physical activity out of health facilities. All participants signed an informed consent in which they agreed to participate to the research study. The study was approved by the institutional expert committee since in accordance research procedures were in accordance with the ethical standards for human experimentation and with the Helsinki Declaration.
HUMAN AND ANIMAL RIGHTS
All research procedures followed were in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national), and with the Helsinki Declaration of 1975, as revised in 2008 (http://www.wma.net/en/20activities/10ethics/10helsinki/)
CONSENT FOR PUBLICATION
Not applicable.
CONFLICT OF INTEREST
AA, MM, EM, SF, and LB declare their partnership to the academic spin-off B.M.E.D. SRL (Bio-Medical Engineering Development, Department of Information Engineering, Polytechnic University of Marche, Ancona, Italy, www.bmed-bioengineering.com). The remaining authors have no financial and/or personal relationships with people or organizations that could inappropriately bias this work.
ACKNOWLEDGEMENTS
AA was responsible for study design and manuscript writing. MM, AS, EM, LM, FDN, SF were responsible for programming, data acquisition and analysis, and manuscript draft revising. LB conceived the study, managed the group and critically revised the manuscript.


